Rapid GAL gene switch of Saccharomyces cerevisiae depends on nuclear Gal3, not nucleocytoplasmic trafficking of Gal3 and Gal80
- PMID: 21890741
- PMCID: PMC3213366
- DOI: 10.1534/genetics.111.131839
Rapid GAL gene switch of Saccharomyces cerevisiae depends on nuclear Gal3, not nucleocytoplasmic trafficking of Gal3 and Gal80
Abstract
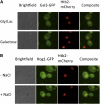
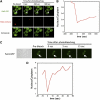
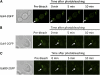
The yeast transcriptional activator Gal4 localizes to UAS(GAL) sites even in the absence of galactose but cannot activate transcription due to an association with the Gal80 protein. By 4 min after galactose addition, Gal4-activated gene transcription ensues. It is well established that this rapid induction arises through a galactose-triggered association between the Gal80 and Gal3 proteins that decreases the association of Gal80 and Gal4. How this happens mechanistically remains unclear. Strikingly different hypotheses prevail concerning the possible roles of nucleocytoplasmic distribution and trafficking of Gal3 and Gal80 and where in the cell the initial Gal3-Gal80 association occurs. Here we tested two conflicting hypotheses by evaluating the subcellular distribution and dynamics of Gal3 and Gal80 with reference to induction kinetics. We determined that the rates of nucleocytoplasmic trafficking for both Gal80 and Gal3 are slow relative to the rate of induction. We find that depletion of the nuclear pool of Gal3 slows the induction kinetics. Thus, nuclear Gal3 is critical for rapid induction. Fluorescence-recovery-after-photobleaching experiments provided data suggesting that the Gal80-Gal4 complex exhibits kinetic stability in the absence of galactose. Finally, we detect Gal3 at the UAS(GAL) only if Gal80 is covalently linked to the DNA-binding domain. Taken altogether, these new findings lead us to propose that a transient interaction of Gal3 with Gal4-associated Gal80 could explain the rapid response of this system. This notion could also explain earlier observations.
Figures

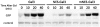



References
-
- Blackwell E., Halatek I. M., Kim H. J. N., Ellicott A. T., Obukhov A. A., et al. , 2003. Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol. Cell. Biol. 23: 1135–1150 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

