The oil spill in ageing Bruch membrane
- PMID: 21890786
- PMCID: PMC3633599
- DOI: 10.1136/bjophthalmol-2011-300344
The oil spill in ageing Bruch membrane
Abstract
Ageing is the largest risk factor for age-related macular degeneration (AMD), and soft drusen and basal linear deposits are lipid-rich extracellular lesions specific to AMD. Oil red O binding neutral lipid represents a major age-related deposition in the Bruch membrane (BrM) and the first identified druse component. Decades after these seminal observations, a natural history of neutral lipid deposition has been articulated and a biochemical model proposed. Results obtained with multiple biochemical, histochemical, and ultrastructural methods, and supported indirectly by epidemiology, suggest that the RPE secretes apolipoprotein B (apoB)-lipoprotein particles of unusual composition into BrM, where they accumulate with age eventually forming a lipid wall, a precursor of basal linear deposit. The authors propose that constituents of these lesions interact with reactive oxygen species to form pro-inflammatory peroxidised lipids that elicit neovascularisation. Here, the authors summarise key evidence supporting both accumulation of BrM lipoproteins leading to lesion formation and lipoprotein production by the RPE. The authors update their model with genetic associations between AMD and genes historically associated with plasma HDL metabolism, and suggest future directions for research and therapeutic strategies based on an oil-spill analogy.
Figures



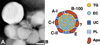
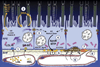
References
-
- Miller JW. Treatment of age-related macular degeneration: beyond VEG-F. Jpn J Ophthalmol. 2010;54:523–528. - PubMed
-
- Pauleikhoff D, Harper CA, Marshall J, et al. Aging changes in Bruch’s membrane: a histochemical and morphological study. Ophthalmology. 1990;97:171–178. - PubMed
-
- Sheraidah G, Steinmetz R, Maguire J, et al. Correlation between lipids extracted from Bruch’s membrane and age. Ophthalmology. 1993;100:47–51. - PubMed
-
- Holz FG, Sheraidah G, Pauleikhoff D, et al. Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch Ophthalmol. 1994;112:402–406. - PubMed
-
- Wolter JR, Falls HF. Bilateral confluent drusen. Arch Ophthalmol. 1962;68:219–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
