Protein phosphatase Pph3 and its regulatory subunit Psy2 regulate Rad53 dephosphorylation and cell morphogenesis during recovery from DNA damage in Candida albicans
- PMID: 21890819
- PMCID: PMC3209060
- DOI: 10.1128/EC.05042-11
Protein phosphatase Pph3 and its regulatory subunit Psy2 regulate Rad53 dephosphorylation and cell morphogenesis during recovery from DNA damage in Candida albicans
Abstract
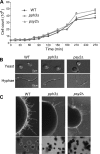
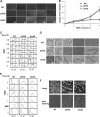
The ability of the pathogenic fungus Candida albicans to switch cellular morphologies is important for infection and virulence. Recent studies have revealed that C. albicans yeast cells can switch to filamentous growth under genotoxic stress in a manner dependent on the DNA replication/damage checkpoint. Here, we have investigated the functions of Pph3 (orf19.4378) and Psy2 (orf19.3685), whose orthologues in Saccharomyces cerevisiae mediate the dephosphorylation of the DNA damage checkpoint kinase Rad53 and the histone variant H2AX during recovery from DNA damage. Deleting PPH3 or PSY2 causes hypersensitivity to DNA-damaging agents, including cisplatin, methylmethane sulfonate (MMS), and UV light. In addition, pph3Δ and psy2Δ cells exhibit strong filamentous growth under genotoxic stress. Flow cytometry analysis shows that the mutant cells have lost the ability to adapt to genotoxic stress and remain arrested even after the stress is withdrawn. Furthermore, we show that Pph3 and Psy2 are required for the dephosphorylation of Rad53, but not H2AX, during DNA damage recovery. Taken together, these results show that C. albicans Pph3 and Psy2 have important roles in mediating genotoxin-induced filamentous growth and regulating Rad53 dephosphorylation.
Figures




Similar articles
-
Pph3 dephosphorylation of Rad53 is required for cell recovery from MMS-induced DNA damage in Candida albicans.PLoS One. 2012;7(5):e37246. doi: 10.1371/journal.pone.0037246. Epub 2012 May 14. PLoS One. 2012. PMID: 22606354 Free PMC article.
-
Characterization of Pph3-mediated dephosphorylation of Rad53 during methyl methanesulfonate-induced DNA damage repair in Candida albicans.Biochem J. 2017 Mar 23;474(7):1293-1306. doi: 10.1042/BCJ20160889. Biochem J. 2017. PMID: 28183985
-
Pph3-Psy2 is a phosphatase complex required for Rad53 dephosphorylation and replication fork restart during recovery from DNA damage.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9290-5. doi: 10.1073/pnas.0703252104. Epub 2007 May 21. Proc Natl Acad Sci U S A. 2007. PMID: 17517611 Free PMC article.
-
Phosphatases, DNA damage checkpoints and checkpoint deactivation.Cell Cycle. 2007 Dec 15;6(24):3058-64. doi: 10.4161/cc.6.24.5100. Epub 2007 Sep 20. Cell Cycle. 2007. PMID: 18075314 Review.
-
DNA damage checkpoint and repair: From the budding yeast Saccharomyces cerevisiae to the pathogenic fungus Candida albicans.Comput Struct Biotechnol J. 2021 Nov 25;19:6343-6354. doi: 10.1016/j.csbj.2021.11.033. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34938410 Free PMC article. Review.
Cited by
-
Hydroxyurea treatment inhibits proliferation of Cryptococcus neoformans in mice.Front Microbiol. 2012 May 24;3:187. doi: 10.3389/fmicb.2012.00187. eCollection 2012. Front Microbiol. 2012. PMID: 22783238 Free PMC article.
-
Ppg1, a PP2A-type protein phosphatase, controls filament extension and virulence in Candida albicans.Eukaryot Cell. 2014 Dec;13(12):1538-47. doi: 10.1128/EC.00199-14. Epub 2014 Oct 17. Eukaryot Cell. 2014. PMID: 25326520 Free PMC article.
-
Ser/Thr protein phosphatases in fungi: structure, regulation and function.Microb Cell. 2019 Apr 24;6(5):217-256. doi: 10.15698/mic2019.05.677. Microb Cell. 2019. PMID: 31114794 Free PMC article. Review.
-
Loss of Gst1 enhances resistance to MMS by reprogramming the transcription of DNA damage response genes in a Rad53-dependent manner in Candida albicans.Cell Commun Signal. 2024 Oct 14;22(1):495. doi: 10.1186/s12964-024-01865-7. Cell Commun Signal. 2024. PMID: 39402632 Free PMC article.
-
Loss of Arp1, a putative actin-related protein, triggers filamentous and invasive growth and impairs pathogenicity in Candida albicans.Comput Struct Biotechnol J. 2020 Dec 1;18:4002-4015. doi: 10.1016/j.csbj.2020.11.034. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33363697 Free PMC article.
References
-
- Andaluz E., Ciudad T., Gomez-Raja J., Calderone R., Larriba G. 2006. Rad52 depletion in Candida albicans triggers both the DNA-damage checkpoint and filamentation accompanied by but independent of expression of hypha-specific genes. Mol. Microbiol. 59:1452–1472 - PubMed
-
- Bachewich C., Nantel A., Whiteway M. 2005. Cell cycle arrest during S or M phase generates polarized growth via distinct signals in Candida albicans. Mol. Microbiol. 57:942–959 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

