Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA
- PMID: 21890902
- PMCID: PMC3241667
- DOI: 10.1093/nar/gkr695
Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA
Abstract
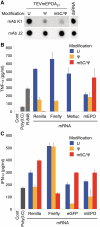
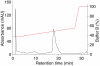
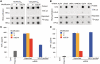
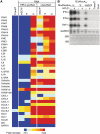
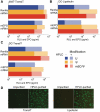
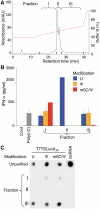
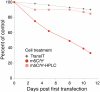
In vitro-transcribed mRNA has great therapeutic potential to transiently express the encoded protein without the adverse effects of viral and DNA-based constructs. Mammalian cells, however, contain RNA sensors of the innate immune system that must be considered in the generation of therapeutic RNA. Incorporation of modified nucleosides both reduces innate immune activation and increases translation of mRNA, but residual induction of type I interferons (IFNs) and proinflammatory cytokines remains. We identify that contaminants, including double-stranded RNA, in nucleoside-modified in vitro-transcribed RNA are responsible for innate immune activation and their removal by high performance liquid chromatography (HPLC) results in mRNA that does not induce IFNs and inflammatory cytokines and is translated at 10- to 1000-fold greater levels in primary cells. Although unmodified mRNAs were translated significantly better following purification, they still induced high levels of cytokine secretion. HPLC purified nucleoside-modified mRNA is a powerful vector for applications ranging from ex vivo stem cell generation to in vivo gene therapy.
Figures
References
-
- Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010;394:189–193. - PubMed
-
- Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

