A second base pair interaction between U3 small nucleolar RNA and the 5'-ETS region is required for early cleavage of the yeast pre-ribosomal RNA
- PMID: 21890904
- PMCID: PMC3239212
- DOI: 10.1093/nar/gkr675
A second base pair interaction between U3 small nucleolar RNA and the 5'-ETS region is required for early cleavage of the yeast pre-ribosomal RNA
Abstract
In eukaryotes, U3 snoRNA is essential for pre-rRNA maturation. Its 5'-domain was found to form base pair interactions with the 18S and 5'-ETS parts of the pre-rRNA. In Xenopus laevis, two segments of U3 snoRNA form base-pair interactions with the 5'-ETS region and only one of them is essential to the maturation process. In Saccharomyces cerevisiae, two similar U3 snoRNA-5' ETS interactions are possible; but, the functional importance of only one of them had been tested. Surprisingly, this interaction, which corresponds to the non-essential one in X. laevis, is essential for cell growth and pre-rRNA maturation in yeast. In parallel with [Dutca et al. (2011) The initial U3 snoRNA:pre-rRNA base pairing interaction required for pre-18S rRNA folding revealed by in vivo chemical probing. Nucleic Acids Research, 39, 5164-5180], here we show, that the second possible 11-bp long interaction between the 5' domain of S. cerevisiae U3 snoRNA and the pre-rRNA 5'-ETS region (helix VI) is also essential for pre-rRNA processing and cell growth. Compensatory mutations in one-half of helix VI fully restored cell growth. Only a partial restoration of growth was obtained upon extension of compensatory mutations to the entire helix VI, suggesting sequence requirement for binding of specific proteins. Accordingly, we got strong evidences for a role of segment VI in the association of proteins Mpp10, Imp4 and Imp3.
© The Author(s) 2011. Published by Oxford University Press.
Figures
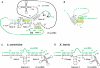

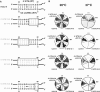
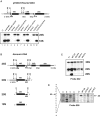
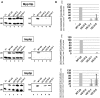
 percentages). The values given are mean values of three independent experiments and their standard deviations are represented by bars.
percentages). The values given are mean values of three independent experiments and their standard deviations are represented by bars.Similar articles
-
Imp3 unfolds stem structures in pre-rRNA and U3 snoRNA to form a duplex essential for small subunit processing.RNA. 2013 Oct;19(10):1372-83. doi: 10.1261/rna.039511.113. Epub 2013 Aug 26. RNA. 2013. PMID: 23980203 Free PMC article.
-
Xenopus U3 snoRNA docks on pre-rRNA through a novel base-pairing interaction.RNA. 2004 Jun;10(6):942-53. doi: 10.1261/rna.5256704. RNA. 2004. PMID: 15146078 Free PMC article.
-
An evolutionary intra-molecular shift in the preferred U3 snoRNA binding site on pre-ribosomal RNA.Nucleic Acids Res. 2005 Sep 6;33(15):4995-5005. doi: 10.1093/nar/gki815. Print 2005. Nucleic Acids Res. 2005. PMID: 16147982 Free PMC article.
-
Mpp10p, a new protein component of the U3 snoRNP required for processing of 18S rRNA precursors.Nucleic Acids Symp Ser. 1997;(36):64-7. Nucleic Acids Symp Ser. 1997. PMID: 9478208 Review.
-
Inside the 40S ribosome assembly machinery.Curr Opin Chem Biol. 2011 Oct;15(5):657-63. doi: 10.1016/j.cbpa.2011.07.023. Epub 2011 Aug 20. Curr Opin Chem Biol. 2011. PMID: 21862385 Free PMC article. Review.
Cited by
-
Stage-specific assembly events of the 6-MDa small-subunit processome initiate eukaryotic ribosome biogenesis.Nat Struct Mol Biol. 2015 Nov;22(11):920-3. doi: 10.1038/nsmb.3111. Epub 2015 Oct 19. Nat Struct Mol Biol. 2015. PMID: 26479197
-
DEAD-box RNA helicase 10 is required for 18S rRNA maturation by controlling the release of U3 snoRNA from pre-rRNA in embryonic stem cells.Nat Commun. 2024 Nov 27;15(1):10303. doi: 10.1038/s41467-024-53822-0. Nat Commun. 2024. PMID: 39604362 Free PMC article.
-
Emerging Data on the Diversity of Molecular Mechanisms Involving C/D snoRNAs.Noncoding RNA. 2021 May 6;7(2):30. doi: 10.3390/ncrna7020030. Noncoding RNA. 2021. PMID: 34066559 Free PMC article. Review.
-
Eukaryotic Ribosome Biogenesis: The 40S Subunit.Acta Naturae. 2022 Jan-Mar;14(1):14-30. doi: 10.32607/actanaturae.11540. Acta Naturae. 2022. PMID: 35441050 Free PMC article.
-
Molecular architecture of the 90S small subunit pre-ribosome.Elife. 2017 Feb 28;6:e22086. doi: 10.7554/eLife.22086. Elife. 2017. PMID: 28244370 Free PMC article.
References
-
- Venema J, Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999;33:261–311. - PubMed
-
- Bachellerie JP, Cavaille J. Guiding ribose methylation of rRNA. Trends Biochem. Sci. 1997;22:257–261. - PubMed
-
- Maxwell ES, Fournier MJ. The small nucleolar RNAs. Annu. Rev. Biochem. 1995;64:897–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

