Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years
- PMID: 21892005
- PMCID: PMC3225769
- DOI: 10.4161/hv.7.9.15999
Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years
Abstract
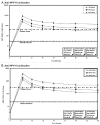
The HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Biologicals) has been shown to induce a robust immune response in women aged 15-55 years (103514/NCT00196937). This follow-up study is the first report of persistence of immune response and safety profile through 48 months after vaccination in women aged 15-55 years. In this open-label, age-stratified Phase III study in Germany and Poland (105882/NCT00196937), healthy women aged 15-55 years received 3 doses of HPV-16/18 AS04-adjuvanted vaccine at 0, 1, and 6 months. Anti-HPV-16/18 seropositivity rates and geometric mean antibody titers (GMTs) were assessed by enzyme-linked immunosorbent assay (ELISA) in women aged 15-25 (n=168), 26-45 (n=186) and 46-55 years (n=177) from the time of first vaccination through 48 months. At Month 48, all subjects were seropositive for anti-HPV-16 antibodies and 99.4% were seropositive for anti-HPV-18. Antibody kinetics were as previously reported, with peak response at Month 7 followed by a gradual decline tending towards a plateau in all age groups. Anti-HPV-16/18 GMTs were sustained at Month 48 in all age groups, including women aged 46-55 years in whom GMTs were respectively 11-fold and 5-fold higher than natural infection levels. The vaccine exhibited a clinically acceptable safety profile in all age groups. In summary, the HPV-16/18 AS04-adjuvanted vaccine induces high and sustained immune responses in women aged 15-55 years, with antibody levels remaining several-fold higher than natural infection levels for at least 4 years after the first vaccine dose.
Figures


References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
