Different phenotypic consequences of simultaneous versus stepwise Apc loss
- PMID: 21892206
- PMCID: PMC3235266
- DOI: 10.1038/onc.2011.385
Different phenotypic consequences of simultaneous versus stepwise Apc loss
Abstract
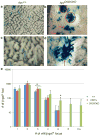
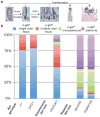
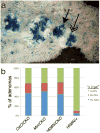
APC is considered a gatekeeper for colorectal cancer (CRC). Cells with heterozygous APC mutations have altered expression profiles suggesting that the first APC hit may help set the stage for subsequent transformation. Therefore, we measured transformation efficiency following what we have designated as 'simultaneous' versus 'stepwise' Apc loss. We combined a conditional Apc allele (Apc(CKO)) with a Cre reporter gene and an out-of-frame Cre allele (Pms2(cre)) that stochastically becomes functional by a frameshift mutation in single cells. Loss of one Apc allele (Apc(CKO/+)) had little consequence, whereas simultaneous loss of both Apc alleles (Apc(CKO/CKO)) resulted in increased clonal expansion (crypt fission), consistent with the gatekeeper function of Apc. Interestingly, our analyses showed that most of the Apc-deficient crypts in Apc(CKO/CKO) mice appeared normal, with morphological transformation, including β-catenin deregulation, occurring in only 17% of such crypts. To determine whether transformation efficiency was different following stepwise Apc loss, we combined Apc(CKO) with a germline mutant allele, either Apc(Min) or Apc(1638N). Transformation efficiency following stepwise Apc loss (Apc(Min/CKO) or Apc(1638N/CKO)) was increased five-fold and essentially all of the Apc-deficient cells were dysplastic. In summary, our data suggest that the gatekeeper function of Apc consists of two roles, clonal expansion and morphological transformation, because simultaneous Apc loss frequently leads to occult clonal expansion without morphological transformation, whereas stepwise Apc loss more often results in visible neoplasia. Finally, that Apc-deficient cells in certain scenarios can retain a normal phenotype is unexpected and may have clinical implications for surveillance strategies to prevent CRC.
Conflict of interest statement
Figures




References
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

