Signal transduction involving the dmp1 transcription factor and its alteration in human cancer
- PMID: 21892281
- PMCID: PMC3161675
- DOI: 10.4137/cmo.s548
Signal transduction involving the dmp1 transcription factor and its alteration in human cancer
Abstract
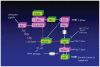
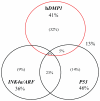
Dmp1 (cyclin D-interacting myb-like protein 1; also called Dmtf1) is a transcription factor that has been isolated in a yeast two-hybrid screen through its binding property to cyclin D2. Dmp1 directly binds to and activates the Arf promoter and induces Arf-p53-dependent cell cycle arrest in primary cells. D-type cyclins usually inhibit Dmp1-mediated transcription in a Cdk-independent fashion; however, Dmp1 shows synergistic effects with D-cyclins on the Arf promoter. Ras or Myc oncogene-induced tumor formation is accelerated in both Dmp1(+/-) and Dmp1(-/-) mice with no significant differences between Dmp1(+/-) and Dmp1(-/-). Thus, Dmp1 is haplo-insufficient for tumor suppression. Tumors from Dmp1(-/-) or Dmp1(+/-) mice often retain wild-type Arf and p53, suggesting that Dmp1 is a physiological regulator of the Arf-p53 pathway. The Dmp1 promoter is activated by oncogenic Ras-Raf signaling, while it is repressed by physiological mitogenic stimuli, overexpression of E2F proteins, and genotoxic stimuli mediated by NF-κB. The human DMP1 gene (hDMP1) is located on chromosome 7q21 and is hemizygously deleted in approximately 40% of human lung cancers, especially those that retain normal INK4a/ARF and P53 loci. Thus, hDMP1 is clearly involved in human carcinogenesis, and tumors with hDMP1 deletion may constitute a discrete disease entity.
Keywords: Arf; Dmp1; Ras; haplo-insufficiency; lung cancer; p53.
Figures

References
-
- Adnane J, Shao Z, Robbins PD. Cyclin D1 associates with the TBP-associated factor TAF(II)250 to regulate Sp1-mediated transcription. Oncogene. 1999;18:239–47. - PubMed
-
- Arnold A, Papanikolau A. Cyclin D1 in breast pathogenesis. J Clin Oncol. 2006;23:4215–24. - PubMed
-
- Bernards R. CDK-independent activities of D type cyclins. Biochem Biophys Acta. 1999;1424(2–3):M17–22. - PubMed
-
- Bieche I, Champeme MH, Matifas F, Hacene K, Callahan R, Lidereau R. Loss of heterozygosity on chromosome 7q and aggressive primary breast cancer. Lancet. 1992;339:139–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

