The proof for new oral anticoagulants: clinical trial evidence
- PMID: 21892362
- PMCID: PMC3150805
- DOI: 10.1007/s12570-011-0063-9
The proof for new oral anticoagulants: clinical trial evidence
Abstract
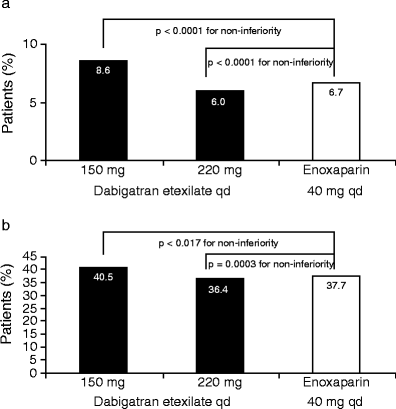
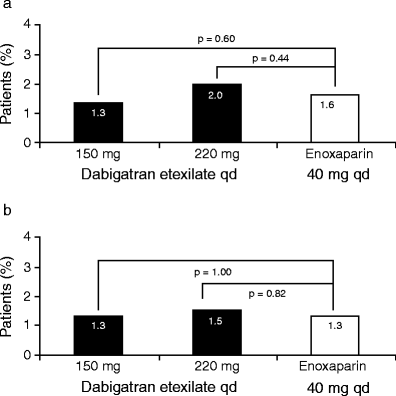
INTRODUCTION: Patients undergoing elective total hip or total knee replacement surgery are at increased risk of venous thromboembolism in the post-operative period and are recommended to receive thromboprophylaxis for 10-35 days. Although several thromboprophylactic agents are available, these are associated with well-recognized limitations. For the low molecular weight heparins (LMWHs) such as enoxaparin, these limitations include parenteral administration, indirect mode of action, inability to inhibit clot-bound thrombin and association with complications such as heparin-induced thrombocytopenia. These limitations make post-operative thromboprophylaxis challenging. Several new oral anticoagulants are in the advanced stages of clinical development. These agents have been designed to target either thrombin (dabigatran etexilate) or factor Xa (rivaroxaban and apixaban), which are key coagulation cascade enzymes. METHODS AND RESULTS: This review will present the published phase III clinical trial evidence of the efficacy and safety of dabigatran etexilate, rivaroxaban and apixaban, compared with the LMWH enoxaparin for the prevention of venous thromboembolism in patients who have undergone elective total hip or total knee replacement surgery. All three agents have shown comparable or superior efficacy compared with the European dose regimen of enoxaparin (40 mg once daily), and comparable rates of major bleeding events. Dabigatran etexilate and rivaroxaban are currently licensed for use following elective hip and knee replacement surgery in many countries, but no direct comparative data exist upon which to base the choice of agent. CONCLUSION: A thorough assessment of each individual patient's thromboembolic and bleeding risks should be the basis of selecting the agent in order to balance efficacy and safety.
Figures

References
-
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, American College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. - DOI - PubMed
-
- Pradaxa® Summary of Product Characteristics (2009) Boehringer Ingelheim International GmbH. http://emc.medicines.org.uk/medicine/20760. Accessed 10 June 2010
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
