Reverse orthogonal strategy for oligosaccharide synthesis
- PMID: 21892457
- PMCID: PMC3805137
- DOI: 10.1039/c1cc13409d
Reverse orthogonal strategy for oligosaccharide synthesis
Abstract
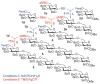
Herein, we report the invention of a novel expeditious concept for oligosaccharide synthesis. Unlike the classic orthogonal strategy based on leaving groups, the reverse approach is based on orthogonal protecting groups, herein p-methoxybenzyl and 4-pentenoyl, which allows for efficient oligosaccharide assembly in the reverse direction.
This journal is © The Royal Society of Chemistry 2011
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

