Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects
- PMID: 21893135
- PMCID: PMC7103316
- DOI: 10.1016/j.addr.2011.08.002
Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects
Abstract
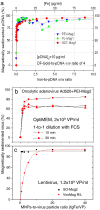
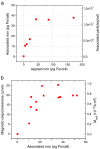
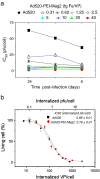
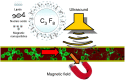
Nucleic acids carry the building plans of living systems. As such, they can be exploited to make cells produce a desired protein, or to shut down the expression of endogenous genes or even to repair defective genes. Hence, nucleic acids are unique substances for research and therapy. To exploit their potential, they need to be delivered into cells which can be a challenging task in many respects. During the last decade, nanomagnetic methods for delivering and targeting nucleic acids have been developed, methods which are often referred to as magnetofection. In this review we summarize the progress and achievements in this field of research. We discuss magnetic formulations of vectors for nucleic acid delivery and their characterization, mechanisms of magnetofection, and the application of magnetofection in viral and nonviral nucleic acid delivery in cell culture and in animal models. We summarize results that have been obtained with using magnetofection in basic research and in preclinical animal models. Finally, we describe some of our recent work and end with some conclusions and perspectives.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures











References
-
- Vaheri A., Pagano J.S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965;27:434–436. - PubMed
-
- Tatum E.L. Molecular biology, nucleic acids, and the future of medicine. Perspect. Biol. Med. 1966;10:19–32. - PubMed
-
- Rosenecker J. The long and winding road to clinical success in gene therapy. Curr. Opin. Mol. Ther. 2010;12:507–508. - PubMed
-
- Gene Therapy Clinical Trials Worldwide Provided by the Journal of Gene Medicine. John Wiley & Sons Ltd; 2010. Gene Therapy Clinical Trials Worldwide.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

