Natural and engineered coding variation in antidepressant-sensitive serotonin transporters
- PMID: 21893166
- PMCID: PMC3850749
- DOI: 10.1016/j.neuroscience.2011.08.056
Natural and engineered coding variation in antidepressant-sensitive serotonin transporters
Abstract
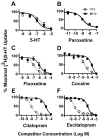
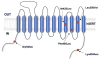
The presynaptic serotonin (5-HT) transporter (SERT) is a key regulator of 5-HT signaling and is a major target for antidepressant medications and psychostimulants. In recent years, studies of natural and engineered genetic variation in SERT have provided new opportunities to understand structural dimensions of drug interactions and regulation of the transporter, to explore 5-HT contributions to antidepressant action, and to assess the impact of SERT-mediated 5-HT contributions to neuropsychiatric disorders. Here we review three examples from our recent studies where genetic changes in SERT, identified or engineered, have led to new models, findings, and theories that cast light on new dimensions of 5-HT action in the CNS and periphery. First, we review our work to identify specific residues through which SERT recognizes antagonists, and the conversion of this knowledge to the creation of mice lacking high-affinity antidepressant and cocaine sensitivity. Second, we discuss our studies of functional coding variation in SERT that exists in commonly used strains of inbred mice, and how this variation is beginning to reveal novel 5-HT-associated phenotypes. Third, we review our identification and functional characterization of multiple, hyperactive SERT coding variants in subjects with autism. Each of these activities has driven the development of new model systems that can be further exploited to understand the contribution of 5-HT signaling to risk for neuropsychiatric disorders and their treatment.
Copyright © 2011. Published by Elsevier Ltd.
Figures
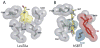
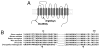
References
-
- Abumaria N, Rygula R, Hiemke C, Fuchs E, Havemann-Reinecke U, Rüther E, Flügge G. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. Eur Neuropsychopharmacol. 2007;17:417–429. - PubMed
-
- Adkins EM, Barker EL, Blakely RD. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition. Mol Pharmacol. 2001;59:514–523. - PubMed
-
- Anderson GM. Genetics of childhood disorders: XLV. Autism, part 4: serotonin in autism. J Am Acad Child Adolesc Psychiatry. 2002;41:1513–1516. - PubMed
-
- Barkan T, Gurwitz D, Levy G, Weizman A, Rehavi M. Biochemical and pharmacological characterization of the serotonin transporter in human peripheral blood lymphocytes. Eur Neuropsychopharmacol. 2004;14:237–243. - PubMed
-
- Barker EL, Perlman MA, Adkins EM, Houlihan WJ, Pristupa ZB, Niznik HB, Blakely RD. High affinity recognition of serotonin transporter antagonists defined by species-scanning mutagenesis. An aromatic residue in transmembrane domain I dictates species-selective recognition of citalopram and mazindol. J Biol Chem. 1998;273:19459–19468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

