Mre11-Rad50 complex crystals suggest molecular calisthenics
- PMID: 21893433
- PMCID: PMC3185151
- DOI: 10.1016/j.dnarep.2011.07.008
Mre11-Rad50 complex crystals suggest molecular calisthenics
Abstract
Recently published crystal structures of different Mre11 and Rad50 complexes show the arrangement of these proteins and imply dramatic ligand-induced rearrangements with important functional consequences.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
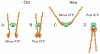
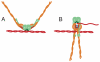

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

