Therapeutic vaccines for chronic diseases: successes and technical challenges
- PMID: 21893545
- PMCID: PMC3146783
- DOI: 10.1098/rstb.2011.0103
Therapeutic vaccines for chronic diseases: successes and technical challenges
Abstract
Chronic, non-communicable diseases are the major cause of death and disability worldwide and have replaced infectious diseases as the major burden of society in large parts of the world. Despite the complexity of chronic diseases, relatively few predisposing risk factors have been identified by the World Health Organization. Those include smoking, alcohol abuse, obesity, high cholesterol and high blood pressure as the cause of many of these chronic conditions. Here, we discuss several examples of vaccines that target these risk factors with the aim of preventing the associated diseases and some of the challenges they face.
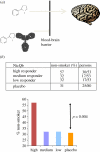
Figures



References
-
- World Health Organization 2003. WHO World Health Report 2002—reducing risks, promoting healthy life, pp. 49–97. See http://www.who.int/whr/2002/en/index.html. - PubMed
-
- Rohn T. A., Bachmann M. F. 2010. Vaccines against non-communicable diseases. Curr. Opin. Immunol. 22, 391–396 10.1016/j.coi.2010.02.009 (doi:10.1016/j.coi.2010.02.009) - DOI - PubMed
-
- Maurer P., Jennings G. T., Willers J., Rohner F., Lindman Y., Roubicek K., Renner W. A., Muller P., Bachmann M. F. 2005. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunol. 35, 2031–2040 10.1002/eji.200526285 (doi:10.1002/eji.200526285) - DOI - PubMed
-
- Johnson J. E., Chiu W. 2000. Structures of virus and virus-like particles. Curr. Opin. Struct. Biol. 10, 229–235 10.1016/S0959-440X(00)00073-7 (doi:10.1016/S0959-440X(00)00073-7) - DOI - PubMed
-
- Pumpens P., Grens E. 1999. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 442, 1–6 10.1016/S0014-5793(98)01599-3 (doi:10.1016/S0014-5793(98)01599-3) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
