Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission
- PMID: 21893600
- PMCID: PMC3171119
- DOI: 10.1083/jcb.201011027
Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission
Abstract
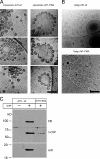
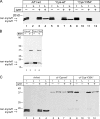
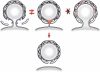
Formation of coated vesicles requires two striking manipulations of the lipid bilayer. First, membrane curvature is induced to drive bud formation. Second, a scission reaction at the bud neck releases the vesicle. Using a reconstituted system for COPI vesicle formation from purified components, we find that a dimerization-deficient Arf1 mutant, which does not display the ability to modulate membrane curvature in vitro or to drive formation of coated vesicles, is able to recruit coatomer to allow formation of COPI-coated buds but does not support scission. Chemical cross-linking of this Arf1 mutant restores vesicle release. These experiments show that initial curvature of the bud is defined primarily by coatomer, whereas the membrane curvature modulating activity of dimeric Arf1 is required for membrane scission.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

