ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway
- PMID: 21894173
- PMCID: PMC3365289
- DOI: 10.1038/icb.2011.71
ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway
Abstract
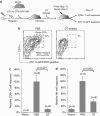
Generating a cytotoxic CD8(+) T-cell response that can eradicate malignant cells is the primary objective of cancer vaccine strategies. In this study we have characterized the innate and adaptive immune response to the ISCOMATRIX adjuvant, and the ability of vaccine antigens formulated with this adjuvant to promote antitumor immunity. ISCOMATRIX adjuvant led to a rapid innate immune cell response at the injection site, followed by the activation of natural killer and dendritic cells (DC) in regional draining lymph nodes. Strikingly, major histocompatibility complex (MHC) class I cross-presentation by CD8α(+) and CD8α(-) DCs was enhanced by up to 100-fold when antigen was formulated with ISCOMATRIX adjuvant. These coordinated features enabled efficient CD8(+) T-cell cross-priming, which exhibited prophylactic and therapeutic tumoricidal activity. The therapeutic efficacy of an ISCOMATRIX vaccine was further improved when co-administered with an anti-CD40 agonist antibody, suggesting that ISCOMATRIX-based vaccines may combine favorably with other immune modifiers in clinical development to treat cancer. Finally, we identified a requirement for the myeloid differentiation primary response gene 88 (MyD88) adapter protein for both innate and adaptive immune responses to ISCOMATRIX vaccines in vivo. Taken together, our findings support the utility of the ISCOMATRIX adjuvant for use in the development of novel vaccines, particularly those requiring strong CD8(+) T-cell immune responses, such as therapeutic cancer vaccines.
Figures

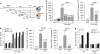




References
-
- Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. - PubMed
-
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. - PubMed
-
- Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–3334. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

