Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis
- PMID: 21894191
- PMCID: PMC3364725
- DOI: 10.1038/cr.2011.148
Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis
Abstract
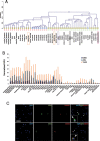
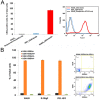
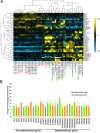
Rapid and dependable methods for isolating human pluripotent stem cell (hPSC) populations are urgently needed for quality control in basic research and in cell-based therapy applications. Using lectin arrays, we analyzed glycoproteins extracted from 26 hPSC samples and 22 differentiated cell samples, and identified a small group of lectins with distinctive binding signatures that were sufficient to distinguish hPSCs from a variety of non-pluripotent cell types. These specific biomarkers were shared by all the 12 human embryonic stem cell and the 14 human induced pluripotent stem cell samples examined, regardless of the laboratory of origin, the culture conditions, the somatic cell type reprogrammed, or the reprogramming method used. We demonstrated a practical application of specific lectin binding by detecting hPSCs within a differentiated cell population with lectin-mediated staining followed by fluorescence microscopy and flow cytometry, and by enriching and purging viable hPSCs from mixed cell populations using lectin-mediated cell separation. Global gene expression analysis showed pluripotency-associated differential expression of specific fucosyltransferases and sialyltransferases, which may underlie these differences in protein glycosylation and lectin binding. Taken together, our results show that protein glycosylation differs considerably between pluripotent and non-pluripotent cells, and demonstrate that lectins may be used as biomarkers to monitor pluripotency in stem cell populations and for removal of viable hPSCs from mixed cell populations.
Figures

Comment in
-
Purging and isolating pluripotent cells, "sweet" dreams become true?Cell Res. 2011 Nov;21(11):1526-7. doi: 10.1038/cr.2011.163. Epub 2011 Oct 25. Cell Res. 2011. PMID: 22025252 Free PMC article. No abstract available.
Similar articles
-
Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells.Sci Rep. 2015 Aug 25;5:13317. doi: 10.1038/srep13317. Sci Rep. 2015. PMID: 26304831 Free PMC article.
-
Live-cell imaging of human pluripotent stem cells by a novel lectin probe rBC2LCN.Methods Mol Biol. 2014;1200:313-8. doi: 10.1007/978-1-4939-1292-6_26. Methods Mol Biol. 2014. PMID: 25117245
-
Expression and Purification of a Human Pluripotent Stem Cell-Specific Lectin Probe, rBC2LCN.Methods Mol Biol. 2020;2132:453-461. doi: 10.1007/978-1-0716-0430-4_44. Methods Mol Biol. 2020. PMID: 32306352
-
Recent Trends in Research with Human Pluripotent Stem Cells: Impact of Research and Use of Cell Lines in Experimental Research and Clinical Trials.Stem Cell Reports. 2018 Aug 14;11(2):485-496. doi: 10.1016/j.stemcr.2018.06.012. Epub 2018 Jul 19. Stem Cell Reports. 2018. PMID: 30033087 Free PMC article. Review.
-
Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle.Int J Mol Sci. 2021 May 9;22(9):5011. doi: 10.3390/ijms22095011. Int J Mol Sci. 2021. PMID: 34065074 Free PMC article. Review.
Cited by
-
Potassium as a pluripotency-associated element identified through inorganic element profiling in human pluripotent stem cells.Sci Rep. 2017 Jul 10;7(1):5005. doi: 10.1038/s41598-017-05117-2. Sci Rep. 2017. PMID: 28694442 Free PMC article.
-
The safety of embryonic stem cell therapy relies on teratoma removal.Oncotarget. 2012 Jan;3(1):7-8. doi: 10.18632/oncotarget.434. Oncotarget. 2012. PMID: 22294556 Free PMC article. No abstract available.
-
A technique for removing tumourigenic pluripotent stem cells using rBC2LCN lectin.Regen Ther. 2020 May 19;14:306-314. doi: 10.1016/j.reth.2020.03.017. eCollection 2020 Jun. Regen Ther. 2020. PMID: 32462059 Free PMC article.
-
Glycans in spent embryo culture medium are related to the implantation ability of blastocysts.Heliyon. 2023 May 12;9(5):e16255. doi: 10.1016/j.heliyon.2023.e16255. eCollection 2023 May. Heliyon. 2023. PMID: 37229168 Free PMC article.
-
Plasma glycomics predict cardiovascular disease in patients with ART-controlled HIV infections.FASEB J. 2019 Feb;33(2):1852-1859. doi: 10.1096/fj.201800923R. Epub 2018 Sep 5. FASEB J. 2019. PMID: 30183373 Free PMC article.
References
-
- Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. - PubMed
-
- Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3:347–361. - PubMed
-
- Kannagi R, Levery SB, Ishigami F, et al. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983;258:8934–8942. - PubMed
-
- Kolle G, Ho M, Zhou Q, et al. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells. 2009;27:2446–2456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

