Molecular origin of electron paramagnetic resonance line shapes on β-barrel membrane proteins: the local solvation environment modulates spin-label configuration
- PMID: 21894979
- PMCID: PMC3199607
- DOI: 10.1021/bi200971x
Molecular origin of electron paramagnetic resonance line shapes on β-barrel membrane proteins: the local solvation environment modulates spin-label configuration
Abstract
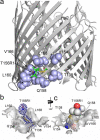
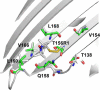
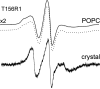
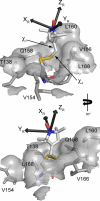
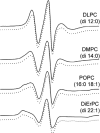
In this work, electron paramagnetic resonance (EPR) spectroscopy and X-ray crystallography were used to examine the origins of EPR line shapes from spin-labels at the protein-lipid interface on the β-barrel membrane protein BtuB. Two atomic-resolution structures were obtained for the methanethiosulfonate spin-label derivatized to cysteines on the membrane-facing surface of BtuB. At one of these sites, position 156, the label side chain resides in a pocket formed by neighboring residues; however, it extends from the protein surface and yields a single-component EPR spectrum in the crystal that results primarily from fast rotation about the fourth and fifth bonds linking the spin-label to the protein backbone. In lipid bilayers, site 156 yields a multicomponent spectrum resulting from different rotameric states of the labeled side chain. Moreover, changes in the lipid environment, such as variations in bilayer thickness, modulate the EPR spectrum by modulating label rotamer populations. At a second site, position 371, the labeled side chain interacts with a pocket on the protein surface, leading to a highly immobilized single-component EPR spectrum that is not sensitive to hydrocarbon thickness. This spectrum is similar to that seen at other sites that are deep in the hydrocarbon, such as position 170. This work indicates that the rotameric states of spin-labels on exposed hydrocarbon sites are sensitive to the environment at the protein-hydrocarbon interface, and that this environment may modulate weak interactions between the labeled side chain and the protein surface. In the case of BtuB, lipid acyl chain packing is not symmetric around the β-barrel, and EPR spectra from labeled hydrocarbon-facing sites in BtuB may reflect this asymmetry. In addition to facilitating the interpretation of EPR spectra of membrane proteins, these results have important implications for the use of long-range distance restraints in protein structure refinement that are obtained from spin-labels.
© 2011 American Chemical Society
Figures





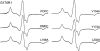
References
-
- Klug CS, Feix JB. Methods and applications of site-directed spin labeling EPR spectroscopy. Methods Cell Biol. 2008;84:617–658. - PubMed
-
- Jeschke G, Polyhach Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Physical Chemistry Chemical Physics. 2007;9:1895–1910. - PubMed
-
- Fanucci GE, Cafiso DS. Recent advances and applications of site-directed spin labeling. Curr Opin Struct Biol. 2006;16:644–653. - PubMed
-
- Hubbell WL, Cafiso DS, Altenbach C. Identifying conformational changes with site-directed spin labeling. Nat Struct Biol. 2000;7:735–739. - PubMed
-
- Hubbell WL, Gross A, Langen R, Lietzow MA. Recent advances in site-directed spin labeling of proteins. Curr Opin Struct Biol. 1998;8:649–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

