Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma
- PMID: 21895629
- PMCID: PMC3415647
- DOI: 10.1111/j.1476-5381.2011.01659.x
Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma
Abstract
Background and purpose: IL-13 is a pleiotropic Th2 cytokine considered likely to play a pivotal role in asthma. Here we describe the preclinical in vitro and in vivo characterization of CAT-354, an IL-13-neutralizing IgG4 monoclonal antibody (mAb), currently in clinical development.
Experimental approach: In vitro the potency, specificity and species selectivity of CAT-354 was assayed in TF-1 cells, human umbilical vein endothelial cells and HDLM-2 cells. The ability of CAT-354 to modulate disease-relevant mechanisms was tested in human cells measuring bronchial smooth muscle calcium flux induced by histamine, eotaxin generation by normal lung fibroblasts, CD23 upregulation in peripheral blood mononuclear cells and IgE production by B cells. In vivo CAT-354 was tested on human IL-13-induced air pouch inflammation in mice, ovalbumin-sensitization and challenge in IL-13 humanized mice and antigen challenge in cynomolgus monkeys.
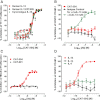
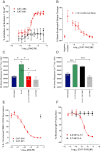
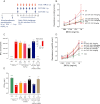
Key results: CAT-354 has a 165 pM affinity for human IL-13 and functionally neutralized human, human variant associated with asthma and atopy (R130Q) and cynomolgus monkey, but not mouse, IL-13. CAT-354 did not neutralize human IL-4. In vitro CAT-354 functionally inhibited IL-13-induced eotaxin production, an analogue of smooth muscle airways hyperresponsiveness, CD23 upregulation and IgE production. In vivo in humanized mouse and cynomolgus monkey antigen challenge models CAT-354 inhibited airways hyperresponsiveness and bronchoalveolar lavage eosinophilia.
Conclusions and implications: CAT-354 is a potent and selective IL-13-neutralizing IgG4 mAb. The preclinical data presented here support the trialling of this mAb in patients with moderate to severe uncontrolled asthma.
© 2011 MedImmune Ltd. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



References
-
- Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096–1103. - PubMed
-
- Bouteiller CL, Astruc R, Minty A, Ferrara P, Lupker JH. Isolation of an IL-13-dependent subclone of the B9 cell line useful for the estimation of human IL-13 bioactivity. J Immunol Methods. 1995;181:29–36. - PubMed
-
- Bree A, Schlerman FJ, Wadanoli M, Tchistiakova L, Marquette K, Tan X-Y, et al. IL-13 blockade reduces lung inflammation after Ascaris suum challenge in cynomolgus monkeys. J Allergy Clin Immunol. 2007;119:1251–1257. - PubMed
-
- Chu HW, Trudeau JB, Balzar S, Wenzel S. Peripheral blood and airway tissue expression of transforming growth factor beta by neutrophils in asthmatic subjects and normal control subjects. J Allergy Clin Immunol. 2000;106:1115–1123. - PubMed
-
- Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–796. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

