Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues
- PMID: 21895961
- PMCID: PMC3215833
- DOI: 10.1111/j.1474-9726.2011.00740.x
Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues
Abstract
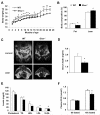
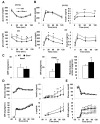
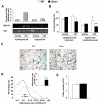
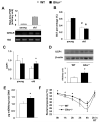
Aging is associated with increased adiposity in white adipose tissues and impaired thermogenesis in brown adipose tissues; both contribute to increased incidences of obesity and type 2 diabetes. Ghrelin is the only known circulating orexigenic hormone that promotes adiposity. In this study, we show that ablation of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R) improves insulin sensitivity during aging. Compared to wild-type (WT) mice, old Ghsr(-/-) mice have reduced fat and preserve a healthier lipid profile. Old Ghsr(-/-) mice also exhibit elevated energy expenditure and resting metabolic rate, yet have similar food intake and locomotor activity. While GHS-R expression in white and brown adipose tissues was below the detectable level in the young mice, GHS-R expression was readily detectable in visceral white fat and interscapular brown fat of the old mice. Gene expression profiles reveal that Ghsr ablation reduced glucose/lipid uptake and lipogenesis in white adipose tissues but increased thermogenic capacity in brown adipose tissues. Ghsr ablation prevents age-associated decline in thermogenic gene expression of uncoupling protein 1 (UCP1). Cell culture studies in brown adipocytes further demonstrate that ghrelin suppresses the expression of adipogenic and thermogenic genes, while GHS-R antagonist abolishes ghrelin's effects and increases UCP1 expression. Hence, GHS-R plays an important role in thermogenic impairment during aging. Ghsr ablation improves aging-associated obesity and insulin resistance by reducing adiposity and increasing thermogenesis. Growth hormone secretagogue receptor antagonists may be a new means of combating obesity by shifting the energy balance from obesogenesis to thermogenesis.
© 2011 The Authors. Aging Cell © 2011 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures


Similar articles
-
aP2-Cre Mediated Ablation of GHS-R Attenuates Adiposity and Improves Insulin Sensitivity during Aging.Int J Mol Sci. 2018 Oct 1;19(10):3002. doi: 10.3390/ijms19103002. Int J Mol Sci. 2018. PMID: 30275401 Free PMC article.
-
Thermogenic characterization of ghrelin receptor null mice.Methods Enzymol. 2012;514:355-70. doi: 10.1016/B978-0-12-381272-8.00022-2. Methods Enzymol. 2012. PMID: 22975064
-
The suppression of ghrelin signaling mitigates age‐associated thermogenic impairment.Aging (Albany NY). 2014 Dec;6(12):1019-32. doi: 10.18632/aging.100706. Aging (Albany NY). 2014. PMID: 25543537 Free PMC article.
-
Genetic variation in brown fat activity and body weight regulation in mice: lessons for human studies.Biochim Biophys Acta. 2014 Mar;1842(3):370-6. doi: 10.1016/j.bbadis.2013.04.025. Epub 2013 May 2. Biochim Biophys Acta. 2014. PMID: 23644163 Review.
-
UCP1: its involvement and utility in obesity.Int J Obes (Lond). 2008 Dec;32 Suppl 7(Suppl 7):S32-8. doi: 10.1038/ijo.2008.236. Int J Obes (Lond). 2008. PMID: 19136989 Free PMC article. Review.
Cited by
-
The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction.PLoS One. 2012;7(2):e32100. doi: 10.1371/journal.pone.0032100. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363801 Free PMC article.
-
β Cell GHS-R Regulates Insulin Secretion and Sensitivity.Int J Mol Sci. 2021 Apr 11;22(8):3950. doi: 10.3390/ijms22083950. Int J Mol Sci. 2021. PMID: 33920473 Free PMC article.
-
Both ghrelin deletion and unacylated ghrelin overexpression preserve muscles in aging mice.Aging (Albany NY). 2020 Jul 26;12(14):13939-13957. doi: 10.18632/aging.103802. Epub 2020 Jul 26. Aging (Albany NY). 2020. PMID: 32712599 Free PMC article.
-
Ghrelin regulation of glucose metabolism.Peptides. 2018 Feb;100:236-242. doi: 10.1016/j.peptides.2017.12.015. Peptides. 2018. PMID: 29412824 Free PMC article. Review.
-
Ghrelin receptor regulates appetite and satiety during aging in mice by regulating meal frequency and portion size but not total food intake.J Nutr. 2014 Sep;144(9):1349-55. doi: 10.3945/jn.114.191171. Epub 2014 Jul 2. J Nutr. 2014. PMID: 24991043 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

