Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma
- PMID: 21896009
- PMCID: PMC3194222
- DOI: 10.1111/j.1365-2567.2011.03473.x
Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma
Abstract
Respiratory viral infections are frequently associated with exacerbations of asthma. Double-stranded RNA (dsRNA) produced during viral infections may be one of the stimuli for exacerbation. We aimed to assess the potential effect of dsRNA on certain aspects of chronic asthma through the administration of polyinosine-polycytidylic acid (poly I:C), synthetic dsRNA, to a rat model of asthma. Brown Norway rats were sensitized to ovalbumin and challenged three times to evoke airway remodelling. The effect of poly I:C on the ovalbumin-induced airway inflammation and structural changes was assessed from bronchoalveolar lavage fluid and histological findings. The expression of cytokines and chemokines was evaluated by real-time quantitative reverse transcription PCR and ELISA. Ovalbumin-challenged animals showed an increased number of total cells and eosinophils in bronchoalveolar lavage fluid compared with PBS-challenged controls. Ovalbumin-challenged animals treated with poly I:C showed an increased number of total cells and neutrophils in bronchoalveolar lavage fluid compared with those without poly I:C treatment. Ovalbumin-challenged animals showed goblet cell hyperplasia, increased airway smooth muscle mass, and proliferation of both airway epithelial cells and airway smooth muscle cells. Treatment with poly I:C enhanced these structural changes. Among the cytokines and chemokines examined, the expression of interleukins 12 and 17 and of transforming growth factor-β(1) in ovalbumin-challenged animals treated with poly I:C was significantly increased compared with those of the other groups. Double-stranded RNA enhanced airway inflammation and remodelling in a rat model of bronchial asthma. These observations suggest that viral infections may promote airway remodelling.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures
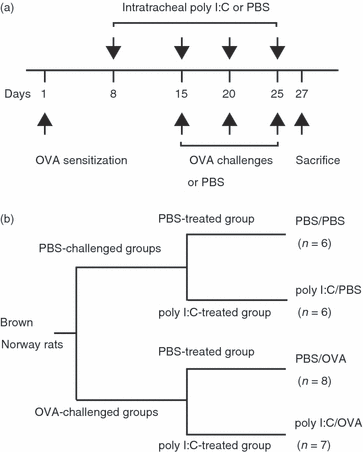

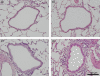
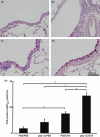
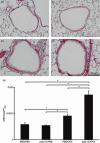
 ) (e). Data are presented as means (SEM). *P < 0·05.
) (e). Data are presented as means (SEM). *P < 0·05.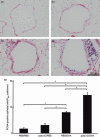
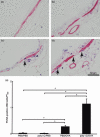
 ) (e). Data are presented as means (SEM). *P < 0·05.
) (e). Data are presented as means (SEM). *P < 0·05.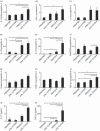
References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. - PubMed
-
- Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–9. - PubMed
-
- Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–9. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. - PubMed

