Leukotriene C(4) prevents the complete maturation of murine dendritic cells and modifies interleukin-12/interleukin-23 balance
- PMID: 21896013
- PMCID: PMC3194226
- DOI: 10.1111/j.1365-2567.2011.03478.x
Leukotriene C(4) prevents the complete maturation of murine dendritic cells and modifies interleukin-12/interleukin-23 balance
Abstract
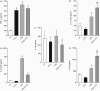
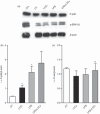
Leukotriene C(4) is an important mediator in the development of inflammatory reactions and ischaemia. Previous studies have shown that leukotriene C(4) is able to modulate the function of dendritic cells (DCs) and induce their chemotaxis from skin to lymph node. In this study, we decided to evaluate the modulation exerted by leukotriene C(4) on DCs, depending on their status of activation. We showed for the first time that leukotriene C(4) stimulates endocytosis both in immature and lipopolysaccharide (LPS) -activated DCs. Moreover, it suppressed the interleukin-12p70 (IL-12p70) release, but induces the secretion of IL-23 by DCs activated with LPS and promotes the expansion of T helper type 17 (Th17) lymphocytes. Furthermore, blocking the release of IL-23 reduced the percentages of CD4(+) T cells producing IL-17 in a mixed lymphocyte reaction. Ours results suggest that leukotriene C(4) interferes with the complete maturation of inflammatory DCs in terms of phenotype and antigen uptake, while favouring the release of IL-23, the main cytokine involved in the maintenance of the Th17 profile.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures
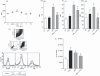
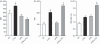

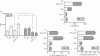
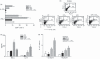
Similar articles
-
Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation.Blood. 2001 Sep 1;98(5):1512-23. doi: 10.1182/blood.v98.5.1512. Blood. 2001. PMID: 11520802
-
High dose lipopolysaccharide triggers polarization of mouse thymic Th17 cells in vitro in the presence of mature dendritic cells.Cell Immunol. 2012;274(1-2):98-108. doi: 10.1016/j.cellimm.2012.01.006. Epub 2012 Feb 6. Cell Immunol. 2012. PMID: 22361175
-
Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways.J Immunol. 2008 Jul 15;181(2):1536-47. doi: 10.4049/jimmunol.181.2.1536. J Immunol. 2008. PMID: 18606709 Free PMC article.
-
Leukotrienes modulate cytokine release from dendritic cells.Immunology. 2005 Dec;116(4):418-28. doi: 10.1111/j.1365-2567.2005.02241.x. Immunology. 2005. PMID: 16313356 Free PMC article.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
Molecular biochemical aspects of salt (sodium chloride) in inflammation and immune response with reference to hypertension and type 2 diabetes mellitus.Lipids Health Dis. 2021 Aug 1;20(1):83. doi: 10.1186/s12944-021-01507-8. Lipids Health Dis. 2021. PMID: 34334139 Free PMC article. Review.
-
Disruption of the alox5ap gene ameliorates focal ischemic stroke: possible consequence of impaired leukotriene biosynthesis.BMC Neurosci. 2012 Nov 30;13:146. doi: 10.1186/1471-2202-13-146. BMC Neurosci. 2012. PMID: 23194405 Free PMC article.
-
Leukotriene C4 induces migration of human monocyte-derived dendritic cells without loss of immunostimulatory function.Blood. 2012 Mar 29;119(13):3113-22. doi: 10.1182/blood-2011-10-385930. Epub 2012 Feb 8. Blood. 2012. PMID: 22323449 Free PMC article.
-
Alleviating Promotion of Inflammation and Cancer Induced by Nonsteroidal Anti-Inflammatory Drugs.Int J Inflam. 2017;2017:9632018. doi: 10.1155/2017/9632018. Epub 2017 May 10. Int J Inflam. 2017. PMID: 28573063 Free PMC article. Review.
References
-
- Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;32:2325–30. - PubMed
-
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. - PubMed
-
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. - PubMed
-
- Reis e Sousa C. Activation of dendritic cells: translating innate into adaptative immunity. Curr Opin Immunol. 2006;16:21–5. - PubMed
-
- Sabatté J, Maggini J, Nahmod K, et al. Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev. 2007;18:5–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

