Methyl-binding domain protein-based DNA isolation from human blood serum combines DNA analyses and serum-autoantibody testing
- PMID: 21896199
- PMCID: PMC3180258
- DOI: 10.1186/1472-6890-11-11
Methyl-binding domain protein-based DNA isolation from human blood serum combines DNA analyses and serum-autoantibody testing
Abstract
Background: Circulating cell free DNA in serum as well as serum-autoantibodies and the serum proteome have great potential to contribute to early cancer diagnostics via non invasive blood tests. However, most DNA preparation protocols destroy the protein fraction and therefore do not allow subsequent protein analyses. In this study a novel approach based on methyl binding domain protein (MBD) is described to overcome the technical difficulties of combining DNA and protein analysis out of one single serum sample.
Methods: Serum or plasma samples from 98 control individuals and 54 breast cancer patients were evaluated upon silica membrane- or MBD affinity-based DNA isolation via qPCR targeting potential DNA methylation markers as well as by protein-microarrays for tumor-autoantibody testing.
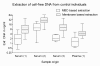
Results: In control individuals, an average DNA level of 22.8 ± 25.7 ng/ml was detected applying the silica membrane based protocol and 8.5 ± 7.5 ng/ml using the MBD-approach, both values strongly dependent on the serum sample preparation methods used. In contrast to malignant and benign tumor serum samples, cell free DNA concentrations were significantly elevated in sera of metastasizing breast cancer patients. Technical evaluation revealed that serum upon MBD-based DNA isolation is suitable for protein-array analyses when data are consistent to untreated serum samples.
Conclusion: MBD affinity purification allows DNA isolations under native conditions retaining the protein function, thus for example enabling combined analyses of DNA methylation and autoantigene-profiles from the same serum sample and thereby improving minimal invasive diagnostics.
Figures
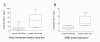


Similar articles
-
A novel method to capture methylated human DNA from stool: implications for colorectal cancer screening.Clin Chem. 2007 Sep;53(9):1646-51. doi: 10.1373/clinchem.2007.086223. Clin Chem. 2007. PMID: 17712002
-
A novel sensitive detection method for DNA methylation in circulating free DNA of pancreatic cancer.PLoS One. 2020 Jun 10;15(6):e0233782. doi: 10.1371/journal.pone.0233782. eCollection 2020. PLoS One. 2020. PMID: 32520974 Free PMC article.
-
A MBD-seq protocol for large-scale methylome-wide studies with (very) low amounts of DNA.Epigenetics. 2017 Sep;12(9):743-750. doi: 10.1080/15592294.2017.1335849. Epub 2017 Nov 6. Epigenetics. 2017. PMID: 28703682 Free PMC article.
-
Methyl-CpG-binding domain proteins: readers of the epigenome.Epigenomics. 2015;7(6):1051-73. doi: 10.2217/epi.15.39. Epub 2015 Apr 30. Epigenomics. 2015. PMID: 25927341 Review.
-
Cancer immunomics using autoantibody signatures for biomarker discovery.Mol Cell Proteomics. 2007 Jul;6(7):1115-22. doi: 10.1074/mcp.R600016-MCP200. Epub 2007 Mar 20. Mol Cell Proteomics. 2007. PMID: 17376768 Review.
Cited by
-
Diagnostic Performance of Plasma DNA Methylation Profiles in Lung Cancer, Pulmonary Fibrosis and COPD.EBioMedicine. 2015 Jul 2;2(8):929-36. doi: 10.1016/j.ebiom.2015.06.025. eCollection 2015 Aug. EBioMedicine. 2015. PMID: 26425700 Free PMC article. Clinical Trial.
-
Cytosine 5-Hydroxymethylation of the LZTS1 Gene Is Reduced in Breast Cancer.Transl Oncol. 2013 Dec 1;6(6):715-21. doi: 10.1593/tlo.13523. eCollection 2013 Dec 1. Transl Oncol. 2013. PMID: 24466374 Free PMC article.
References
-
- Dobrzycka B, Terlikowski SJ, Mazurek A, Kowalczuk O, Niklinska W, Chyczewski L, Kulikowski M. Circulating free DNA, p53 antibody and mutations of KRAS gene in endometrial cancer. Int J Cancer. 2009. - PubMed
-
- Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer--a survey. Biochim Biophys Acta. 2007;1775:181–232. - PubMed
-
- Wang BG, Huang HY, Chen YC, Bristow RE, Kassauei K, Cheng CC, Roden R, Sokoll LJ, Chan DW, Shih I. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003;63:3966–3968. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

