Cysteine cathepsins S and L modulate anti-angiogenic activities of human endostatin
- PMID: 21896479
- PMCID: PMC3199463
- DOI: 10.1074/jbc.M111.284869
Cysteine cathepsins S and L modulate anti-angiogenic activities of human endostatin
Abstract
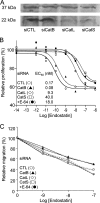
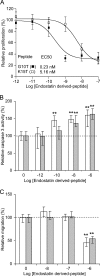
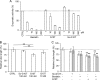
Human endostatin, a potent anti-angiogenic protein, is generated by release of the C terminus of collagen XVIII. Here, we propose that cysteine cathepsins are involved in both the liberation and activation of bioactive endostatin fragments, thus regulating their anti-angiogenic properties. Cathepsins B, S, and L efficiently cleaved in vitro FRET peptides that encompass the hinge region corresponding to the N terminus of endostatin. However, in human umbilical vein endothelial cell-based assays, silencing of cathepsins S and L, but not cathepsin B, impaired the generation of the ∼22-kDa endostatin species. Moreover, cathepsins L and S released two peptides from endostatin with increased angiostatic properties and both encompassing the NGR sequence, a vasculature homing motif. The G10T peptide (residues 1455-1464: collagen XVIII numbering) displayed compelling anti-proliferative (EC(50) = 0.23 nm) and proapoptotic properties. G10T inhibited aminopeptidase N (APN/CD13) and reduced tube formation of endothelial cells in a manner similar to bestatin. Combination of G10T with bestatin resulted in no further increase in anti-angiogenic activity. Taken together, these data suggest that endostatin-derived peptides may represent novel molecular links between cathepsins and APN/CD13 in the regulation of angiogenesis.
Figures


References
-
- O'Reilly M. S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W. S., Flynn E., Birkhead J. R., Olsen B. R., Folkman J. (1997) Cell 88, 277–285 - PubMed
-
- Marneros A. G., Olsen B. R. (2001) Matrix Biol. 20, 337–345 - PubMed
-
- Ling Y., Yang Y., Lu N., You Q. D., Wang S., Gao Y., Chen Y., Guo Q. L. (2007) Biochem. Biophys. Res. Commun. 361, 79–84 - PubMed
-
- Dhanabal M., Volk R., Ramchandran R., Simons M., Sukhatme V. P. (1999) Biochem. Biophys. Res. Commun. 258, 345–352 - PubMed
-
- Dixelius J., Cross M., Matsumoto T., Sasaki T., Timpl R., Claesson-Welsh L. (2002) Cancer Res. 62, 1944–1947 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

