A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration
- PMID: 21896482
- PMCID: PMC3199457
- DOI: 10.1074/jbc.M111.242438
A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration
Abstract
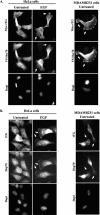
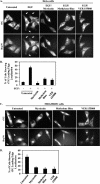
Cell migration is essential for several important biological outcomes and is involved in various developmental disorders and disease states including cancer cell invasiveness and metastasis. A fundamental step in cell migration is the development of a leading edge. By using HeLa carcinoma cells as an initial model system, we uncovered a surprising role for the heat shock protein 70 (Hsp70) and its ability to bind the protein cross-linking enzyme, tissue transglutaminase (tTG), in cancer cell migration. Treatment of HeLa cells with EGF results in the activation of a plasma membrane-associated pool of tTG and its redistribution to the leading edges of these cells, which are essential events for EGF-stimulated HeLa cell migration. However, we then found that the ability of tTG to be localized to the leading edge is dependent on Hsp70. Similarly, the localization of tTG to the leading edges of MDAMB231 breast carcinoma cells, where it also plays an essential role in their migration, has a strict requirement for Hsp70. Treatment of these different cell lines with inhibitors against the ATP hydrolytic activity of Hsp70 prevented tTG from localizing to their leading edges and thereby blocked EGF-stimulated HeLa cell migration, as well as the constitutive migration normally exhibited by MDAMB231 cells. These findings highlight a new and unconventional role for the chaperonin activity of Hsp70 in the localization of a key regulatory protein (tTG) at the leading edges of cancer cells and the important consequences that this holds for their ability to migrate.
Figures






References
-
- Raja, Sivamani K., Garcia M. S., Isseroff R. R. (2007) Front. Biosci. 12, 2849–2868 - PubMed
-
- Cyster J. G. (2005) Annu. Rev. Immunol. 23, 127–159 - PubMed
-
- Aman A., Piotrowski T. (2010) Dev. Biol. 341, 20–33 - PubMed
-
- Luster A. D., Alon R., von Andrian U. H. (2005) Nat. Immunol. 6, 1182–1190 - PubMed
-
- Keller R. (2005) Curr. Opin. Cell Biol. 17, 533–541 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

