Apolipoprotein A-I exerts bactericidal activity against Yersinia enterocolitica serotype O:3
- PMID: 21896489
- PMCID: PMC3207405
- DOI: 10.1074/jbc.M111.249482
Apolipoprotein A-I exerts bactericidal activity against Yersinia enterocolitica serotype O:3
Abstract
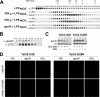
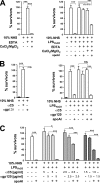
Apolipoprotein A-I (apoA-I), the main protein component of high density lipoprotein (HDL), is well recognized for its antiatherogenic, antioxidant, and antiinflammatory properties. Here, we report a novel role for apoA-I as a host defense molecule that contributes to the complement-mediated killing of an important gastrointestinal pathogen, Gram-negative bacterium Yersinia enterocolitica. We specifically show that the C-terminal domain of apoA-I is the effector site providing the bactericidal activity. Although the presence of the lipopolysaccharide O-antigen on the bacterial surface is absolutely required for apoA-I to kill the bacteria, apoA-I does not interact with the bacteria directly. To the contrary, exposure of the bacteria by serum proteins triggers apoA-I deposition on the bacterial surface. As our data show that both purified lipid-free and HDL-associated apoA-I displays anti-bacterial potential, apoA-I mimetic peptides may be a promising therapeutic agent for the treatment of certain Gram-negative infections.
Figures



References
-
- Singh I. P., Chopra A. K., Coppenhaver D. H., Ananatharamaiah G. M., Baron S. (1999) Antiviral Res. 42, 211–218 - PubMed
-
- Pérez-Morga D., Vanhollebeke B., Paturiaux-Hanocq F., Nolan D. P., Lins L., Homblé F., Vanhamme L., Tebabi P., Pays A., Poelvoorde P., Jacquet A., Brasseur R., Pays E. (2005) Science 309, 469–472 - PubMed
-
- Hubsch A. P., Casas A. T., Doran J. E. (1995) J. Lab. Clin. Med. 126, 548–558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

