Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner
- PMID: 21896734
- PMCID: PMC3179115
- DOI: 10.1073/pnas.1019012108
Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner
Abstract
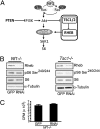
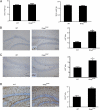
Converging evidence from the analysis of human brain tumors and genetically engineered mice has revealed that the mammalian target of rapamycin (mTOR) pathway is a central regulator of glial and glioma cell growth. In this regard, mutational inactivation of neurofibromatosis-1 (NF1), tuberous sclerosis complex (TSC), and PTEN genes is associated with glioma formation, such that pharmacologic inhibition of mTOR signaling results in attenuated tumor growth. This shared dependence on mTOR suggests that PTEN and NF1 (neurofibromin) glial growth regulation requires TSC/Rheb (Ras homolog enriched in brain) control of mTOR function. In this report, we use a combination of genetic silencing in vitro and conditional mouse transgenesis approaches in vivo to demonstrate that neurofibromin regulates astrocyte cell growth and glioma formation in a TSC/Rheb-independent fashion. First, we show that Nf1 or Pten inactivation, but not Tsc1 loss or Rheb overexpression, increases astrocyte cell growth in vitro. Second, Nf1-deficient increased mTOR signaling and astrocyte hyperproliferation is unaffected by Rheb shRNA silencing. Third, conditional Tsc1 inactivation or Rheb overexpression in glial progenitors of Nf1(+/-) mice does not lead to glioma formation. Collectively, these findings establish TSC/Rheb-independent mechanisms for mTOR-dependent glial cell growth control and gliomagenesis relevant to the design of therapies for individuals with glioma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dan HC, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. - PubMed
-
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–162. - PubMed
-
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

