Targeted killing of a mammalian cell based upon its specialized metabolic state
- PMID: 21896756
- PMCID: PMC3179072
- DOI: 10.1073/pnas.1111312108
Targeted killing of a mammalian cell based upon its specialized metabolic state
Abstract
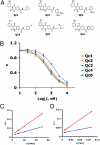
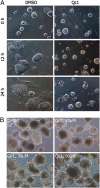
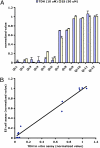
Mouse ES cells use a mitochondrial threonine dehydrogenase (TDH) enzyme to catabolize threonine into glycine and acetyl-CoA. Measurements of mRNA abundance have given evidence that ES cells express upwards of 1,000-fold higher levels of TDH mRNA than any of seven other mouse tissues tested. When cell culture medium is deprived of threonine, ES cells rapidly discontinue DNA synthesis, arrest cell division, and eventually die. Such studies led to the conclusion that mouse ES cells exist in a threonine-dependent metabolic state. Proceeding with the assumption that the active TDH enzyme should be essential for the growth and viability of mouse ES cells, we performed a drug screen in search of specific inhibitors of the purified TDH enzyme. Such efforts led to the discovery of a class of quinazolinecarboxamide (Qc) compounds that inhibit the ability of the TDH enzyme to catabolize threonine into glycine and acetyl-CoA. Administration of Qc inhibitors of TDH to mouse ES cells impeded cell growth and resulted in the induction of autophagy. By contrast, the same chemicals failed to affect the growth of HeLa cells at concentrations 300-fold higher than that required to kill mouse ES cells. It was likewise observed that the Qc class of TDH inhibitors failed to affect the growth or viability of ES cell-derived embryoid body cells known to have extinguished TDH expression. These studies demonstrate how it is possible to kill a specific mammalian cell type on the basis of its specialized metabolic state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Smyth HF, Carpenter CP. Water-soluble derivatives of p-aminobenzene-sulfonamide (sulfanilamide) Science. 1938;87:350–351. - PubMed
-
- Roland S, Ferone R, Harvey RJ, Styles VL, Morrison RW. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J Biol Chem. 1979;254:10337–10345. - PubMed
-
- Carson LE, Campbell CC. The inhibitory effect of three antihistamine compounds on the growth of fungi pathogenic for man. Science. 1950;111:689–691. - PubMed
-
- Nyfeler R, Keller-Schierlein W. [Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: Isolation and structural components] Helv Chim Acta. 1974;57:2459–2477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

