Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation
- PMID: 21896757
- PMCID: PMC3179065
- DOI: 10.1073/pnas.1107533108
Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation
Abstract
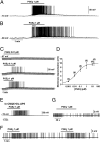
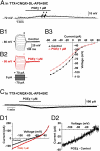
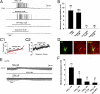
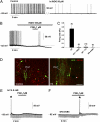
Astrocytes in the hypothalamus release prostaglandin E(2) (PGE(2)) in response to cell-cell signaling initiated by neurons and glial cells. Upon release, PGE(2) stimulates the secretion of gonadotropin-releasing hormone (GnRH), the neuropeptide that controls reproduction, from hypothalamic neuroendocrine neurons. Whether this effect on GnRH secretion is accompanied by changes in the firing behavior of these neurons is unknown. Using patch-clamp recording we demonstrate that PGE(2) exerts a dose-dependent postsynaptic excitatory effect on GnRH neurons. These effects are mimicked by an EP2 receptor agonist and attenuated by protein kinase A (PKA) inhibitors. The acute blockade of prostaglandin synthesis by indomethacin (INDO) or the selective inhibition of astrocyte metabolism by fluoroacetate (FA) suppresses the spontaneous firing activity of GnRH neurons in brain slices. Similarly, GnRH neuronal activity is reduced in mice with impaired astrocytic PGE(2) release due to defective erbB signaling in astrocytes. These results indicate that astrocyte-to-neuron communication in the hypothalamus is essential for the activity of GnRH neurons and suggest that PGE(2) acts as a gliotransmitter within the GnRH neurosecretory system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Panatier A, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

