IRE1-dependent activation of AMPK in response to nitric oxide
- PMID: 21896783
- PMCID: PMC3209336
- DOI: 10.1128/MCB.05668-11
IRE1-dependent activation of AMPK in response to nitric oxide
Abstract
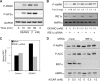
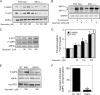
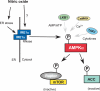
While there can be detrimental consequences of nitric oxide production at pathological concentrations, eukaryotic cells have evolved protective mechanisms to defend themselves against this damage. The unfolded-protein response (UPR), activated by misfolded proteins and oxidative stress, is one adaptive mechanism that is employed to protect cells from stress. Nitric oxide is a potent activator of AMP-activated protein kinase (AMPK), and AMPK participates in the cellular defense against nitric oxide-mediated damage in pancreatic β-cells. In this study, the mechanism of AMPK activation by nitric oxide was explored. The known AMPK kinases LKB1, CaMKK, and TAK1 are not required for the activation of AMPK by nitric oxide. Instead, this activation is dependent on the endoplasmic reticulum (ER) stress-activated protein IRE1. Nitric oxide-induced AMPK phosphorylation and subsequent signaling to AMPK substrates, including Raptor, acetyl coenzyme A carboxylase, and PGC-1α, is attenuated in IRE1α-deficient cells. The endoribonuclease activity of IRE1 appears to be required for AMPK activation in response to nitric oxide. In addition to nitric oxide, stimulation of IRE1 endoribonuclease activity with the flavonol quercetin leads to IRE1-dependent AMPK activation. These findings indicate that the RNase activity of IRE1 participates in AMPK activation and subsequent signaling through multiple AMPK-dependent pathways in response to nitrosative stress.
Figures



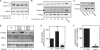
References
-
- Ahn J., Lee H., Kim S., Park J., Ha T. 2008. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 373:545–549 - PubMed
-
- Almeida A., Moncada S., Bolaños J. 2004. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6:45–51 - PubMed
-
- Arnush M., Scarim A., Heitmeier M., Kelly C., Corbett J. 1998. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J. Immunol. 160:2684–2691 - PubMed
-
- Bhaskar P. T., Hay N. 2007. The two TORCs and Akt. Dev. Cell 12:487–502 - PubMed
-
- Calabrese V., et al. 2009. Nitric oxide in cell survival: a janus molecule. Antioxid. Redox. Signal. 11:2717–2739 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
