Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants
- PMID: 21896911
- PMCID: PMC3232794
- DOI: 10.1128/AAC.00544-11
Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants
Abstract
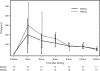
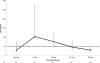
Tenofovir (TFV) is effective in preventing simian immunodeficiency virus (SIV) transmission in a macaque model, is available as the oral agent tenofovir disoproxil fumarate (TDF), and may be useful in the prevention of mother-to-child transmission of human immunodeficiency virus (HIV). We conducted a trial of TDF and TDF-emtricitabine (FTC) in HIV-infected pregnant women and their infants. Women received a single dose of either 600 mg TDF, 900 mg TDF, or 900 mg TDF-600 mg FTC at labor onset or prior to a cesarean section. Infants received no drug or a single dose of TDF at 4 mg/kg of body weight or of TDF at 4 mg/kg plus FTC at 3 mg/kg as soon as possible after birth. All regimens were safe and well tolerated. Maternal areas under the serum concentration-time curve (AUC) and concentrations at the end of sampling after 24 h (C(24)) were similar between the two doses of TDF; the maximum concentrations of the drugs in serum (C(max)) and cord blood concentrations were higher in women delivering via cesarean section than in those who delivered vaginally (P = 0.04 and 0.046, respectively). The median ratio of the TFV concentration in cord blood to that in the maternal plasma at delivery was 0.73 (range, 0.26 to 1.95). Without TDF administration, infants had a median TFV concentration of 12 ng/ml 12 h after birth. Following administration of a single dose of TDF at 4 mg/kg, infant TFV concentrations fell below the targeted level, 50 ng/ml, by 24 h postdose. In HIV-infected pregnant women and their infants, 600 mg of TDF is acceptable as a single dose during labor. Low concentrations at birth support infant dosing as soon after birth as possible. Rapidly decreasing TFV levels in infants suggest that multiple or higher doses of TDF will be necessary to maintain concentrations that are effective for viral suppression.
Figures

References
-
- Best B. M., et al. 2008. High-dose lopinavir and standard-dose emtricitabine pharmacokinetics during pregnancy and postpartum, abstr. 629. Abstr. 15th Conf. Retrovir. Oppor. Infect., Boston, MA
-
- Blum M. R., Chittick G. E., Begley J. A., Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47: 751–759 - PubMed
-
- Chi B. H., et al. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370: 1698–1705 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- N01-DK-9-001/HHSN267200800001C/DK/NIDDK NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01AI068632/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

