5-Aminoimidazole-4-carboxyamide-ribonucleoside (AICAR)-stimulated hepatic expression of Cyp4a10, Cyp4a14, Cyp4a31, and other peroxisome proliferator-activated receptor α-responsive mouse genes is AICAR 5'-monophosphate-dependent and AMP-activated protein kinase-independent
- PMID: 21896918
- PMCID: PMC3226363
- DOI: 10.1124/jpet.111.184242
5-Aminoimidazole-4-carboxyamide-ribonucleoside (AICAR)-stimulated hepatic expression of Cyp4a10, Cyp4a14, Cyp4a31, and other peroxisome proliferator-activated receptor α-responsive mouse genes is AICAR 5'-monophosphate-dependent and AMP-activated protein kinase-independent
Abstract
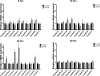
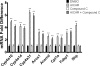
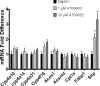
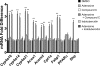
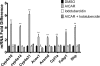
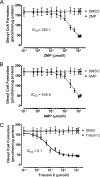
5-Aminoimidazole-4-carboxyamide-ribonucleoside (AICAR), a prodrug activator of AMP-activated protein kinase (AMPK), increased hepatic expression of cytochrome P450 4a10, 4a14, and 4a31 mRNAs 2-, 3-, and 4-fold, respectively, and liver microsomal lauric acid ω-hydroxylation increased 2.8-fold. Likewise, mRNA levels of the peroxisome proliferator-activated receptor α (PPARα)-responsive genes, Acox1, Acadm, Cpt1a, and Fabp1, were also increased by AICAR treatment. AICAR did not elicit these changes in PPARα null mice. In isolated murine hepatocytes, AICAR and adenosine produced similar effects, and these responses were blocked by the PPARα antagonist [(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester (GW6471). Inhibition of AMPK using compound C (dorsomorphin or 6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine) did not block the induction of the PPARα-responsive genes by AICAR or adenosine, and 6,7-dihydro-4-hydroxy-3-(2'-hydroxy[1,1'-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5-carbonitrile (A-769662), a non-nucleoside, direct activator of AMPK, did not increase expression of PPARα-responsive genes. An inhibitor of adenosine kinase, 5-iodotubercidin, blocked these responses, suggesting that the phosphorylation of AICAR and adenosine to AICAR 5'-monophosphate (ZMP) or AMP, respectively, was required. Concentrations of ZMP and AMP were elevated and ATP levels diminished at 24 h. The PPARα-dependent responses were associated with increased concentrations of oleic acid, a potent PPARα agonist, and diminished levels of oleoyl-CoA. Oleoyl-CoA synthase activity was inhibited by ZMP and AMP with IC(50) values of 0.28 and 0.41 mM, respectively. These results suggest that PPARα is activated by increased concentrations of free fatty acids that may arise from impaired fatty acid metabolism caused by altered levels of ATP, AMP, and ZMP after AICAR or adenosine treatment.
Figures
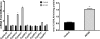
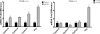
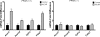
Similar articles
-
Genistein, resveratrol, and 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside induce cytochrome P450 4F2 expression through an AMP-activated protein kinase-dependent pathway.J Pharmacol Exp Ther. 2011 Apr;337(1):125-36. doi: 10.1124/jpet.110.175851. Epub 2011 Jan 4. J Pharmacol Exp Ther. 2011. PMID: 21205922 Free PMC article.
-
Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase.Biochem J. 2004 Dec 1;384(Pt 2):295-305. doi: 10.1042/BJ20040955. Biochem J. 2004. PMID: 15312046 Free PMC article.
-
5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells?Eur J Biochem. 1995 Apr 15;229(2):558-65. doi: 10.1111/j.1432-1033.1995.tb20498.x. Eur J Biochem. 1995. PMID: 7744080
-
AMPK activation enhances PPARα activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway.Arch Biochem Biophys. 2011 Jul;511(1-2):1-7. doi: 10.1016/j.abb.2011.04.010. Epub 2011 Apr 21. Arch Biochem Biophys. 2011. PMID: 21530483
-
Effects of dibutyl phthalate on lipid metabolism in liver and hepatocytes based on PPARα/SREBP-1c/FAS/GPAT/AMPK signal pathway.Food Chem Toxicol. 2021 Mar;149:112029. doi: 10.1016/j.fct.2021.112029. Epub 2021 Jan 26. Food Chem Toxicol. 2021. PMID: 33508418
Cited by
-
AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle.J Physiol. 2013 Oct 15;591(20):5207-20. doi: 10.1113/jphysiol.2013.259515. Epub 2013 Aug 5. J Physiol. 2013. PMID: 23918774 Free PMC article.
-
The Functions of Cytochrome P450 ω-hydroxylases and the Associated Eicosanoids in Inflammation-Related Diseases.Front Pharmacol. 2021 Sep 14;12:716801. doi: 10.3389/fphar.2021.716801. eCollection 2021. Front Pharmacol. 2021. PMID: 34594219 Free PMC article. Review.
-
Crystal structure, vibrational and optic properties of 2-N-methylamino-3-methylpyridine N-oxide - Its X-ray and spectroscopic studies as well as DFT quantum chemical calculations.J Mol Struct. 2019 Nov 5;1195:208-219. doi: 10.1016/j.molstruc.2019.05.064. Epub 2019 May 24. J Mol Struct. 2019. PMID: 32336784 Free PMC article.
-
Structure and Optical Properties of New 2-N-Phenylamino-methyl-nitro-pyridine Isomers.Int J Mol Sci. 2025 Mar 21;26(7):2874. doi: 10.3390/ijms26072874. Int J Mol Sci. 2025. PMID: 40243469 Free PMC article.
-
Regulation of protein-coding gene and long noncoding RNA pairs in liver of conventional and germ-free mice following oral PBDE exposure.PLoS One. 2018 Aug 1;13(8):e0201387. doi: 10.1371/journal.pone.0201387. eCollection 2018. PLoS One. 2018. PMID: 30067809 Free PMC article.
References
-
- Alexandre A, Rossi CR, Sartorelli L, Siliprandi N. (1969) The action of atractyloside and AMP on long-chain fatty acid oxidation and on the ATP-dependent fatty acid thiokinase. FEBS Lett 3:279–282 - PubMed
-
- Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J, Hsueh WA, Beavo JA, Coleman RA, Bornfeldt KE. (2007) Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-γ-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56:1143–1152 - PMC - PubMed
-
- Aymerich I, Foufelle F, Ferré P, Casado FJ, Pastor-Anglada M. (2006) Extracellular adenosine activates AMP-dependent protein kinase (AMPK). J Cell Sci 119:1612–1621 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

