Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer
- PMID: 21897392
- PMCID: PMC3208490
- DOI: 10.1038/bjc.2011.348
Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer
Abstract
Background: Activin A is a multi-functional cytokine belonging to the transforming growth factor-β (TGF-β) superfamily; however, the effect of activin A on angiogenesis remains largely unclear. We found that inhibin β A subunit (INHBA) mRNA is overexpressed in gastric cancer (GC) specimens and investigated the effect of activin A, a homodimer of INHBA, on angiogenesis in GC.
Methods: Anti-angiogenic effects of activin A via p21 induction were evaluated using human umbilical vein endothelial cells (HUVECs) in vitro and a stable INHBA-introduced GC cell line in vivo.
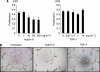
Results: Compared with TGF-β, activin A potently inhibited the cellular proliferation and tube formation of HUVECs with induction of p21. A promoter assay and a chromatin immunoprecipitation assay revealed that activin A directly regulates p21 transcriptional activity through Smads. Stable p21-knockdown significantly enhanced the cellular proliferation of HUVECs. Notably, stable p21-knockdown exhibited a resistance to activin-mediated growth inhibition in HUVECs, indicating that p21 induction has a key role on activin A-mediated growth inhibition in vascular endothelial cells. Finally, a stable INHBA-introduced GC cell line exhibited a decrease in tumour growth and angiogenesis in vivo.
Conclusion: Our findings highlight the suppressive role of activin A, unlike TGF-β, on tumour growth and angiogenesis in GC.
Figures
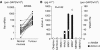





References
-
- Burdette JE, Jeruss JS, Kurley SJ, Lee EJ, Woodruff TK (2005) Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells. Cancer Res 65: 7968–7975 - PubMed
-
- Chen YG, Lui HM, Lin SL, Lee JM, Ying SY (2002) Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 227: 75–87 - PubMed
-
- Dawid IB, Taira M, Good PJ, Rebagliati MR (1992) The role of growth factors in embryonic induction in Xenopus laevis. Mol Reprod Dev 32: 136–144 - PubMed
-
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

