Immunization with aspartate-β-hydroxylase-loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma
- PMID: 21898484
- PMCID: PMC3242918
- DOI: 10.1002/hep.24629
Immunization with aspartate-β-hydroxylase-loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma
Abstract
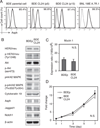
Dendritic cells (DCs) capture and process proteins and present peptides on the cell surface in the context of major histocompatibility complex I and II molecules to induce antigen-specific T cell immune responses. The aims of this study were to (1) employ an expanded and purified DC population and load them with aspartate-β-hydroxylase (ASPH), a highly expressed tumor-associated cell surface protein, and (2) to determine if immunization induced antitumor effects in an orthotopic rat model of intrahepatic cholangiocarcinoma. Splenocytes were incubated with ASPH-coated beads and passed through a magnetic field to yield an 80% pure DC OX62+ population. This DC subset was stimulated with granulocyte-macrophage colony-stimulating factor, interleukin-4, CD40L, and interferon-γ, resulting in a 40-fold increase in interleukin-12A messenger RNA expression to subsequently generate a T helper 1-type immune response. After incubation with the cytokine cocktail, DCs were found to have matured, as demonstrated by increased expression of CD40, CD80, and CD86 costimulatory molecules. Immunization with ASPH-loaded DCs induced antigen-specific immunity. A clone of the parental tumorigenic rat BDEneu cholangiocyte cell line, designated BDEneu-CL24, was found to have the highest number of cells expressing this surface protein (97%); it maintained the same phenotypic characteristics of the parental cell line and was used to produce intrahepatic tumors in immunocompetent syngeneic Fisher-344 rats. Immunization with ASPH-loaded DCs generated cytotoxicity against cholangiocarcinoma cells in vitro and significantly suppressed intrahepatic tumor growth and metastasis, and was associated with increased CD3+ lymphocyte infiltration into the tumors.
Conclusion: These findings suggest that immunization with ASPH-loaded DCs may constitute a novel therapeutic approach for intrahepatic cholangiocarcinoma, because this protein also appears to be highly conserved and expressed on human hepatobiliary tumors.
Copyright © 2011 American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures



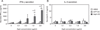


References
-
- Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. - PubMed
-
- Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. - PubMed
-
- Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. - PubMed
-
- Konstadoulakis MM, Roayaie S, Gomatos IP, Labow D, Fiel MI, Miller CM, Schwartz ME. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366–374. - PubMed
-
- Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, Serra C, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 252:107–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
