An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases
- PMID: 21898493
- PMCID: PMC3229841
- DOI: 10.1002/hep.24641
An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases
Figures
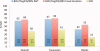
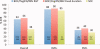



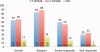
Comment in
-
Rapid virological response: is it four or eight weeks?Hepatology. 2012 Mar;55(3):979; author reply 980-1. doi: 10.1002/hep.25527. Hepatology. 2012. PMID: 22161760 No abstract available.
-
An update on treatment of hepatitis C virus genotype 1 infection and viral load assessments.Hepatology. 2012 Mar;55(3):979-80; author reply 980-1. doi: 10.1002/hep.25516. Hepatology. 2012. PMID: 22161994 No abstract available.
References
-
- Eddy D. A manual for assessing health practices and designing practice guidelines. Philadelphia: American College of Physicians; 1996.
-
- American Gastroenterological Association policy statement on the use of medical practice guidelines by managed care organizations and insurance carriers. Gastroenterology. 1995;108:925–926. - PubMed
-
- American Heart Association. 2011. http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/documents.... Accessed August.
-
- Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139:493–498. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
