The putative Notch ligand HyJagged is a transmembrane protein present in all cell types of adult Hydra and upregulated at the boundary between bud and parent
- PMID: 21899759
- PMCID: PMC3180645
- DOI: 10.1186/1471-2121-12-38
The putative Notch ligand HyJagged is a transmembrane protein present in all cell types of adult Hydra and upregulated at the boundary between bud and parent
Abstract
Background: The Notch signalling pathway is conserved in pre-bilaterian animals. In the Cnidarian Hydra it is involved in interstitial stem cell differentiation and in boundary formation during budding. Experimental evidence suggests that in Hydra Notch is activated by presenilin through proteolytic cleavage at the S3 site as in all animals. However, the endogenous ligand for HvNotch has not been described yet.
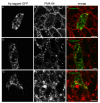
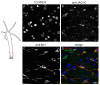
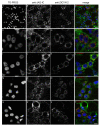
Results: We have cloned a cDNA from Hydra, which encodes a bona-fide Notch ligand with a conserved domain structure similar to that of Jagged-like Notch ligands from other animals. Hyjagged mRNA is undetectable in adult Hydra by in situ hybridisation but is strongly upregulated and easily visible at the border between bud and parent shortly before bud detachment. In contrast, HyJagged protein is found in all cell types of an adult hydra, where it localises to membranes and endosomes. Co-localisation experiments showed that it is present in the same cells as HvNotch, however not always in the same membrane structures.
Conclusions: The putative Notch ligand HyJagged is conserved in Cnidarians. Together with HvNotch it may be involved in the formation of the parent-bud boundary in Hydra. Moreover, protein distribution of both, HvNotch receptor and HyJagged indicate a more widespread function for these two transmembrane proteins in the adult hydra, which may be regulated by additional factors, possibly involving endocytic pathways.
Figures










References
-
- Steele RE. Developmental Signaling in Hydra: What does it take to build a „simple" animal? Developmental Biology. 1996;248:199–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

