Actin and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells
- PMID: 21899882
- PMCID: PMC3193180
- DOI: 10.1016/j.biomaterials.2011.08.059
Actin and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells
Abstract
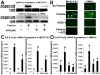
Wear particles at the host bone-implant interface are a major challenge for successful bone implant arthoplasties. Current understanding of aseptic loosening consists of macrophage-mediated inflammatory responses and increasing osteoclastogenesis, which lead to an imbalance between bone formation and resorption. Despite its significant role in bone regeneration and implant osteointegration, the osteoprogenitor response to wear particles has been examined recent years. More specifically, the intracellular mechanism of osteoprogenitor mediated inflammation has not been fully elucidated. In this study, we examined the role of osteoprogenitors and the cellular mechanism by which metal wear particles elicit an inflammatory cascade. Through both in vivo and in vitro experiments, we have demonstrated that osteoprogenitor cells are capable of initiating inflammatory responses by phagocytosing wear particles, which lead to subsequent accumulation of macrophages and osteoclastogenesis, and the ERK_CEBP/β intracellular signaling is a key inflammatory pathway that links phagocytosis of wear particles to inflammatory gene expression in osteoprogenitors. AZD6244 treatment, a potent inhibitor of the ERK pathway, attenuated particle mediated inflammatory osteolysis both in vivo and in vitro. This study advances our understanding of the mechanisms of osteoprogenitor-mediated inflammation, and provides further evidence that the ERK_CEBP/β pathway may be a suitable therapeutic target in the treatment of inflammatory osteolysis.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures







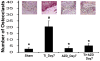

References
-
- Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001:71–7. - PubMed
-
- Schmalzried TP, Callaghan JJ. Wear in total hip and knee replacements. J Bone Joint Surg Am. 1999;81:115–36. - PubMed
-
- Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001:66–70. - PubMed
-
- Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res. 2002;59:507–15. - PubMed
-
- Allen MJ, Myer BJ, Millett PJ, Rushton N. The effects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J Bone Joint Surg Br. 1997;79:475–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

