Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism
- PMID: 21900249
- PMCID: PMC3207440
- DOI: 10.1074/jbc.M111.252171
Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism
Abstract
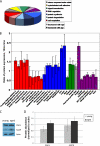
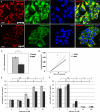
Although neurogenesis occurs in discrete areas of the adult mammalian brain, neural progenitor cells (NPCs) produce fewer new neurons with age. To characterize the molecular changes that occur during aging, we performed a proteomic comparison between primary-cultured NPCs from the young adult and aged mouse forebrain. This analysis yielded changes in proteins necessary for cellular metabolism. Mitochondrial quantity and oxygen consumption rates decrease with aging, although mitochondrial DNA in aged NPCs does not have increased mutation rates. In addition, aged cells are resistant to the mitochondrial inhibitor rotenone and proliferate in response to lowered oxygen conditions. These results demonstrate that aging NPCs display an altered metabolic phenotype, characterized by a coordinated shift in protein expression, subcellular structure, and metabolic physiology.
Figures




References
-
- Jin K., Sun Y., Xie L., Batteur S., Mao X. O., Smelick C., Logvinova A., Greenberg D. A. (2003) Aging Cell 2, 175–183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

