The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons
- PMID: 21900250
- PMCID: PMC3199506
- DOI: 10.1074/jbc.M111.268029
The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons
Abstract
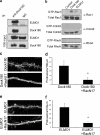
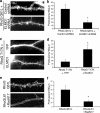
Dendritic spines are actin-rich structures, the formation and plasticity of which are regulated by the Rho GTPases in response to synaptic input. Although several guanine nucleotide exchange factors (GEFs) have been implicated in spine development and plasticity in hippocampal neurons, it is not known how many different Rho GEFs contribute to spine morphogenesis or how they coordinate the initiation, establishment, and maintenance of spines. In this study, we screened 70 rat Rho GEFs in cultured hippocampal neurons by RNA interference and identified a number of candidates that affected spine morphogenesis. Of these, Dock180, which plays a pivotal role in a variety of cellular processes including cell migration and phagocytosis, was further investigated. We show that depletion of Dock180 inhibits spine morphogenesis, whereas overexpression of Dock180 promotes spine morphogenesis. ELMO1, a protein necessary for in vivo functions of Dock180, functions in a complex with Dock180 in spine morphogenesis through activating the Rac GTPase. Moreover, RhoG, which functions upstream of the ELMO1/Dock180 complex, is also important for spine formation. Together, our findings uncover a role for the RhoG/ELMO1/Dock180 signaling module in spine morphogenesis in hippocampal neurons.
Figures




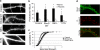
References
-
- Hering H., Sheng M. (2001) Nat. Rev. Neurosci. 2, 880–888 - PubMed
-
- Sheng M., Hoogenraad C. C. (2007) Annu. Rev. Biochem. 76, 823–847 - PubMed
-
- Fiala J. C., Spacek J., Harris K. M. (2002) Brain Res. Brain Res. Rev. 39, 29–54 - PubMed
-
- Bonhoeffer T., Yuste R. (2002) Neuron 35, 1019–1027 - PubMed
-
- Cingolani L. A., Goda Y. (2008) Nat. Rev. Neurosci. 9, 344–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

