A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model
- PMID: 21900387
- PMCID: PMC3227107
- DOI: 10.1101/gr.116764.110
A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model
Abstract
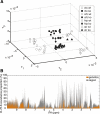
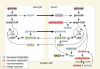
Fragile X syndrome (FXS) is the first cause of inherited intellectual disability, due to the silencing of the X-linked Fragile X Mental Retardation 1 gene encoding the RNA-binding protein FMRP. While extensive studies have focused on the cellular and molecular basis of FXS, neither human Fragile X patients nor the mouse model of FXS--the Fmr1-null mouse--have been profiled systematically at the metabolic and neurochemical level to provide a complementary perspective on the current, yet scattered, knowledge of FXS. Using proton high-resolution magic angle spinning nuclear magnetic resonance ((1)H HR-MAS NMR)-based metabolic profiling, we have identified a metabolic signature and biomarkers associated with FXS in various brain regions of Fmr1-deficient mice. Our study highlights for the first time that Fmr1 gene inactivation has profound, albeit coordinated consequences in brain metabolism leading to alterations in: (1) neurotransmitter levels, (2) osmoregulation, (3) energy metabolism, and (4) oxidative stress response. To functionally connect Fmr1-deficiency to its metabolic biomarkers, we derived a functional interaction network based on the existing knowledge (literature and databases) and show that the FXS metabolic response is initiated by distinct mRNA targets and proteins interacting with FMRP, and then relayed by numerous regulatory proteins. This novel "integrated metabolome and interactome mapping" (iMIM) approach advantageously unifies novel metabolic findings with previously unrelated knowledge and highlights the contribution of novel cellular pathways to the pathophysiology of FXS. These metabolomic and integrative systems biology strategies will contribute to the development of potential drug targets and novel therapeutic interventions, which will eventually benefit FXS patients.
Figures





References
-
- Akerman CJ, Cline HT 2007. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci 30: 382–389 - PubMed
-
- Bardoni B, Davidovic L, Bensaid M, Khandjian EW 2006. The Fragile X syndrome: Exploring its molecular basis and seeking a treatment. Expert Rev Mol Med 8: 1–16 - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R 2007. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 87: 1215–1284 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
