WT1-interacting protein (Wtip) regulates podocyte phenotype by cell-cell and cell-matrix contact reorganization
- PMID: 21900451
- PMCID: PMC3251346
- DOI: 10.1152/ajprenal.00419.2011
WT1-interacting protein (Wtip) regulates podocyte phenotype by cell-cell and cell-matrix contact reorganization
Abstract
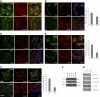
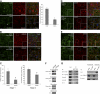
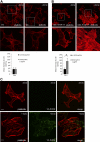
Podocytes respond to environmental cues by remodeling their slit diaphragms and cell-matrix adhesive junctions. Wt1-interacting protein (Wtip), an Ajuba family LIM domain scaffold protein expressed in the podocyte, coordinates cell adhesion changes and transcriptional responses to regulate podocyte phenotypic plasticity. We evaluated effects of Wtip on podocyte cell-cell and cell-matrix contact organization using gain-of- and loss-of-function methods. Endogenous Wtip targeted to focal adhesions in adherent but isolated podocytes and then shifted to adherens junctions after cells made stable, homotypic contacts. Podocytes with Wtip knockdown (shWtip) adhered but failed to spread normally. Noncontacted shWtip podocytes did not assemble actin stress fibers, and their focal adhesions failed to mature. As shWtip podocytes established cell-cell contacts, stable adherens junctions failed to form and F-actin structures were disordered. In shWtip cells, cadherin and β-catenin clustered in irregularly distributed spots that failed to laterally expand. Cell surface biotinylation showed diminished plasma membrane cadherin, β-catenin, and α-catenin in shWtip podocytes, although protein expression was similar in shWtip and control cells. Since normal actin dynamics are required for organization of adherens junctions and focal adhesions, we determined whether Wtip regulates F-actin assembly. Undifferentiated podocytes did not elaborate F-actin stress fibers, but when induced to overexpress WTIP, formed abundant stress fibers, a process blocked by the RhoA inhibitor C3 toxin and a RhoA kinase inhibitor. WTIP directly interacted with Rho guanine nucleotide exchange factor (GEF) 12 (Arhgef12), a RhoA-specific GEF enriched in the glomerulus. In conclusion, stable assembly of podocyte adherens junctions and cell-matrix contacts requires Wtip, a process that may be mediated by spatiotemporal regulation of RhoA activity through appropriate targeting of Arhgef12.
Figures





References
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997 - PubMed
-
- Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol 8: 74–85, 1996 - PubMed
-
- Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet 14: 156–162, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

