Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS
- PMID: 21900512
- PMCID: PMC3234085
- DOI: 10.1152/jn.00312.2011
Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS
Abstract
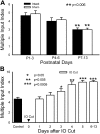
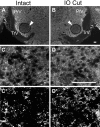
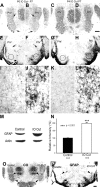
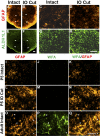
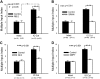
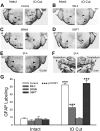
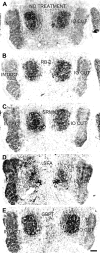
Neonatal damage to the trigeminal nerve leads to "reactive synaptogenesis" in the brain stem sensory trigeminal nuclei. In vitro models of brain injury-induced synaptogenesis have implicated an important role for astrocytes. In this study we tested the role of astrocyte function in reactive synaptogenesis in the trigeminal principal nucleus (PrV) of neonatal rats following unilateral transection of the infraorbital (IO) branch of the trigeminal nerve. We used electrophysiological multiple input index analysis (MII) to estimate the number of central trigeminal afferent fibers that converge onto single barrelette neurons. In the developing PrV, about 30% of afferent connections are eliminated within 2 postnatal weeks. After neonatal IO nerve damage, multiple trigeminal inputs (2.7 times that of the normal inputs) converge on single barrelette cells within 3-5 days; they remain stable up to the second postnatal week. Astrocyte proliferation and upregulation of astrocyte-specific proteins (GFAP and ALDH1L1) accompany reactive synaptogenesis in the IO nerve projection zone of the PrV. Pharmacological blockade of astrocyte function, purinergic receptors, and thrombospondins significantly reduced or eliminated reactive synaptogenesis without changing the MII in the intact PrV. GFAP immunohistochemistry further supported these electrophysiological results. We conclude that immature astrocytes, purinergic receptors, and thrombospondins play an important role in reactive synaptogenesis in the peripherally deafferented neonatal PrV.
Figures

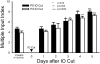
Similar articles
-
Neonatal infraorbital nerve crush-induced CNS synaptic plasticity and functional recovery.J Neurophysiol. 2014 Apr;111(8):1590-600. doi: 10.1152/jn.00658.2013. Epub 2014 Jan 29. J Neurophysiol. 2014. PMID: 24478162 Free PMC article.
-
Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro.J Neurosci. 2012 Sep 19;32(38):13100-10. doi: 10.1523/JNEUROSCI.2604-12.2012. J Neurosci. 2012. PMID: 22993427 Free PMC article.
-
Neonatal deafferentation does not alter membrane properties of trigeminal nucleus principalis neurons.J Neurophysiol. 2001 Mar;85(3):1088-96. doi: 10.1152/jn.2001.85.3.1088. J Neurophysiol. 2001. PMID: 11247979 Free PMC article.
-
Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus.Dev Neurosci. 2016;38(3):163-170. doi: 10.1159/000446395. Epub 2016 Jun 9. Dev Neurosci. 2016. PMID: 27287019 Free PMC article. Review.
-
Neonatal sensory nerve injury-induced synaptic plasticity in the trigeminal principal sensory nucleus.Exp Neurol. 2016 Jan;275 Pt 2(0 2):245-52. doi: 10.1016/j.expneurol.2015.04.022. Epub 2015 May 6. Exp Neurol. 2016. PMID: 25956829 Free PMC article. Review.
Cited by
-
Synaptic ultrastructure changes in trigeminocervical complex posttrigeminal nerve injury.J Comp Neurol. 2016 Feb 1;524(2):309-22. doi: 10.1002/cne.23844. Epub 2015 Jul 16. J Comp Neurol. 2016. PMID: 26132987 Free PMC article.
-
Neonatal infraorbital nerve crush-induced CNS synaptic plasticity and functional recovery.J Neurophysiol. 2014 Apr;111(8):1590-600. doi: 10.1152/jn.00658.2013. Epub 2014 Jan 29. J Neurophysiol. 2014. PMID: 24478162 Free PMC article.
-
Astrocyte derived TSP2 contributes to synaptic alteration and visual dysfunction in retinal ischemia/reperfusion injury.Cell Biosci. 2022 Dec 5;12(1):196. doi: 10.1186/s13578-022-00932-1. Cell Biosci. 2022. PMID: 36471420 Free PMC article.
-
Regulatory effects of inhibiting the activation of glial cells on retinal synaptic plasticity.Neural Regen Res. 2014 Feb 15;9(4):385-93. doi: 10.4103/1673-5374.128240. Neural Regen Res. 2014. PMID: 25206825 Free PMC article.
-
AMPA-silent synapses in brain development and pathology.Nat Rev Neurosci. 2013 Dec;14(12):839-50. doi: 10.1038/nrn3642. Epub 2013 Nov 8. Nat Rev Neurosci. 2013. PMID: 24201185 Review.
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 - PMC - PubMed
-
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 17: 25–51, 2001 - PubMed
-
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15: 542–548, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

