Concomitant administration of Mycobacterium bovis BCG with the meningococcal C conjugate vaccine to neonatal mice enhances antibody response and protective efficacy
- PMID: 21900528
- PMCID: PMC3209029
- DOI: 10.1128/CVI.05247-11
Concomitant administration of Mycobacterium bovis BCG with the meningococcal C conjugate vaccine to neonatal mice enhances antibody response and protective efficacy
Abstract
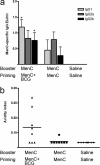
Mycobacterium bovis BCG is administered to human neonates in many countries worldwide. The objective of the study was to assess if BCG could act as an adjuvant for polysaccharide-protein conjugate vaccines in newborns and thereby induce protective immunity against encapsulated bacteria in early infancy when susceptibility is high. We assessed whether BCG could enhance immune responses to a meningococcal C (MenC) conjugate vaccine, MenC-CRM(197), in mice primed as neonates, broaden the antibody response from a dominant IgG1 toward a mixed IgG1 and IgG2a/IgG2b response, and increase protective efficacy, as measured by serum bactericidal activity (SBA). Two-week-old mice were primed subcutaneously (s.c.) with MenC-CRM(197). BCG was administered concomitantly, a day or a week before MenC-CRM(197). An adjuvant effect of BCG was observed only when it was given concomitantly with MenC-CRM(197), with increased IgG response (P = 0.002) and SBA (8-fold) after a second immunization with MenC-CRM(197) without BCG, indicating increased T-cell help. In neonatal mice (1 week old) primed s.c. with MenC-CRM(197) together with BCG, MenC-polysaccharide (PS)-specific IgG was enhanced compared to MenC-CRM(197) alone (P = 0.0015). Sixteen days after the second immunization with MenC-CRM(197), increased IgG (P < 0.05), IgG1 (P < 0.05), IgG2a (P = 0.06), and IgG2b (P < 0.05) were observed, and only mice primed with MenC-CRM(197) plus BCG showed affinity maturation and detectable SBA (SBA > 128). Thus, vaccination with a meningococcal conjugate vaccine (and possibly with other conjugates) may benefit from concomitant administration of BCG in the neonatal period to accelerate and enhance production of protective antibodies, compared to the current infant administration of conjugate which follows BCG vaccination at birth.
Figures


References
-
- Andersen P., Doherty T. M. 2005. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656–662 - PubMed
-
- Avery D. T., Bryant V. L., Ma C. S., de Waal Malefyt R., Tangye S. G. 2008. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 181:1767–1779 - PubMed
-
- Bjarnarson S. P., et al. 2005. The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur. J. Immunol. 35:1037–1045 - PubMed
-
- Borrow R., Balmer P., Miller E. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23:2222–2227 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

