Immunization of mice with Lactobacillus casei expressing a beta-intimin fragment reduces intestinal colonization by Citrobacter rodentium
- PMID: 21900533
- PMCID: PMC3209022
- DOI: 10.1128/CVI.05262-11
Immunization of mice with Lactobacillus casei expressing a beta-intimin fragment reduces intestinal colonization by Citrobacter rodentium
Abstract
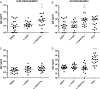
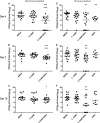
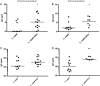
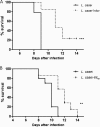
Enteropathogenic Escherichia coli (EPEC) is a common cause of diarrhea in children from developing countries. Intimate adhesion of the bacteria to intestinal cells occurs via binding of the adhesin intimin to the TIR receptor exposed on cell surfaces. Here, Lactobacillus casei expressing a fragment of β-intimin (L. casei-Int(cv)) was tested as mucosal vaccines in mice against intestinal colonization with the murine pathogen Citrobacter rodentium. Oral or sublingual immunization of C57BL/6 mice with L. casei-Int(cv) induced anti-Int(cv) IgA in feces but no IgG in sera. Conversely, anti-Int(cv) IgG was induced in the sera of mice after sublingual immunization with purified Int(cv). All vaccines were able to decrease C. rodentium recovery from feces. However, this reduction was more evident and sustained over time in mice immunized with L. casei-Int(cv) by the sublingual route. These mice also displayed an increase in interleukin 6 (IL-6) and gamma interferon (IFN-γ) secretion by spleen cells 10 days after infection. Additionally, oral or sublingual immunization of C3H/HePas mice, which are highly susceptible to C. rodentium infection, with L. casei-Int(cv) induced anti-Int(cv) antibodies and significantly increased survival after challenge. Immunohistological analysis of colon sections revealed that C. rodentium was located in deep fractions of the tissue from C3H/HePas mice immunized with L. casei whereas superficial staining was observed in colon sections from mice immunized with L. casei-Int(cv.) The results indicate that vaccines composed of L. casei expressing intimin may represent a promising approach and that the C3H/HePas infection model with C. rodentium can be used to evaluate potential vaccines against EPEC.
Figures

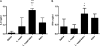

References
-
- Abe C. M., et al. 2009. Virulence features of atypical enteropathogenic Escherichia coli identified by the eae(+) EAF-negative stx(-) genetic profile. Diagn. Microbiol. Infect. Dis. 64:357–365 - PubMed
-
- Adachi K., et al. 2010. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 28:2810–2817 - PubMed
-
- Aida Y., Pabst M. J. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191–195 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

