Fukutin-related protein alters the deposition of laminin in the eye and brain
- PMID: 21900571
- PMCID: PMC6623409
- DOI: 10.1523/JNEUROSCI.2301-11.2011
Fukutin-related protein alters the deposition of laminin in the eye and brain
Abstract
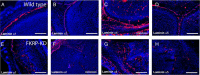
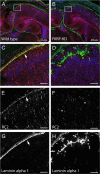
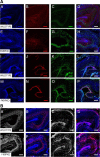
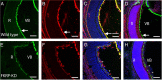
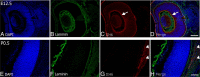
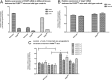
Mutations in fukutin-related protein (FKRP) are responsible for a common group of muscular dystrophies ranging from adult onset limb girdle muscular dystrophies to severe congenital forms with associated structural brain involvement. The defining feature of this group of disorders is the hypoglycosylation of α-dystroglycan and its inability to effectively bind extracellular matrix ligands such as laminin α2. However, α-dystroglycan has the potential to interact with a number of laminin isoforms many of which are basement membrane/tissue specific and developmentally regulated. To further investigate this we evaluated laminin α-chain expression in the cerebral cortex and eye of our FKRP knock-down mouse (FKRP(KD)). These mice showed a marked disturbance in the deposition of laminin α-chains including α1, α2, α4, and α5, although only laminin α1- and γ1-chain mRNA expression was significantly upregulated relative to controls. Moreover, there was a diffuse pattern of laminin deposition below the pial surface which correlated with an abrupt termination of many of the radial glial cells. This along with the pial basement membrane defects, contributed to the abnormal positioning of both early- and late-born neurons. Defects in the inner limiting membrane of the eye were associated with a reduction of laminin α1 demonstrating the involvement of the α-dystroglycan:laminin α1 axis in the disease process. These observations demonstrate for the first time that a reduction in Fkrp influences the ability of tissue-specific forms of α-dystroglycan to direct the deposition of several laminin isoforms in the formation of different basement membranes.
Figures

References
-
- Ackroyd MR, Skordis L, Kaluarachchi M, Godwin J, Prior S, Fidanboylu M, Piercy RJ, Muntoni F, Brown SC. Reduced expression of fukutin related protein in mice results in a model for fukutin related protein associated muscular dystrophies. Brain. 2009;132:439–451. - PubMed
-
- Aumailley M, Pesch M, Tunggal L, Gaill F, Fässler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci. 2000;113:259–268. - PubMed
-
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. - PubMed
-
- Beltrán-Valero de Bernabé D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
