Mitochondrial Ca(2+) uptake is essential for synaptic plasticity in pain
- PMID: 21900577
- PMCID: PMC3179262
- DOI: 10.1523/JNEUROSCI.3093-11.2011
Mitochondrial Ca(2+) uptake is essential for synaptic plasticity in pain
Abstract
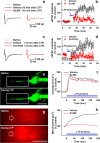
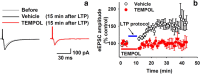
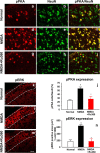
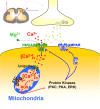
The increase of cytosolic free Ca(2+) ([Ca(2+)](c)) due to NMDA receptor activation is a key step for spinal cord synaptic plasticity by altering cellular signal transduction pathways. We focus on this plasticity as a cause of persistent pain. To provide a mechanism for these classic findings, we report that [Ca(2+)](c) does not trigger synaptic plasticity directly but must first enter into mitochondria. Interfering with mitochondrial Ca(2+) uptake during a [Ca(2+)](c) increase blocks induction of behavioral hyperalgesia and accompanying downstream cell signaling, with reduction of spinal long-term potentiation (LTP). Furthermore, reducing the accompanying mitochondrial superoxide levels lessens hyperalgesia and LTP induction. These results indicate that [Ca(2+)](c) requires downstream mitochondrial Ca(2+) uptake with consequent production of reactive oxygen species (ROS) for synaptic plasticity underlying chronic pain. These results suggest modifying mitochondrial Ca(2+) uptake and thus ROS as a type of chronic pain therapy that should also have broader biologic significance.
Figures



References
-
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. - PubMed
-
- Coderre TJ. Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neurosci Lett. 1992;140:181–184. - PubMed
-
- Cox B. Calcium channel blockers and pain therapy. Curr Rev Pain. 2000;4:488–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
