Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege
- PMID: 21900683
- PMCID: PMC3313662
- DOI: 10.1095/biolreprod.110.089425
Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege
Abstract
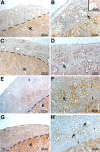
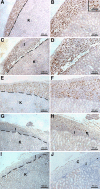
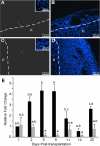
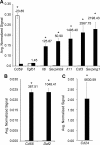
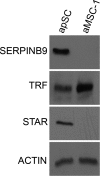
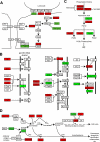
Primary Sertoli cells isolated from mouse testes survive when transplanted across immunological barriers and protect cotransplanted allogeneic and xenogeneic cells from rejection in rodent models. In contrast, the mouse Sertoli cell line (MSC-1) lacks immunoprotective properties associated with primary Sertoli cells. In this study, enriched primary Sertoli cells or MSC-1 cells were transplanted as allografts into the renal subcapsular area of naive BALB/c mice, and their survival in graft sites was compared. While Sertoli cells were detected within the grafts with 100% graft survival throughout the 20-day study, MSC-1 cells were rejected between 11 and 14 days, with 0% graft survival at 20 days posttransplantation. Nonetheless, the mechanism for primary Sertoli cell survival and immunoprotection remains unresolved. To identify immune factors or functional pathways potentially responsible for immune privilege, gene expression profiles of enriched primary Sertoli cells were compared with those of MSC-1 cells. Microarray analysis identified 2369 genes in enriched primary Sertoli cells that were differentially expressed at ±4-fold or higher levels than in MSC-1 cells. Ontological analyses identified multiple immune pathways, which were used to generate a list of 340 immune-related genes. Three functions were identified in primary Sertoli cells as potentially important for establishing immune privilege: suppression of inflammation by specific cytokines and prostanoid molecules, slowing of leukocyte migration by controlled cell junctions and actin polymerization, and inhibition of complement activation and membrane-associated cell lysis. These results increase our understanding of testicular immune privilege and, in the long-term, could lead to improvements in transplantation success.
Figures
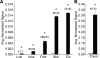

References
-
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol 1977; 25: 1 54 - PubMed
-
- Whitmore WF, III, Karsh L, Gittes RF. The role of germinal epithelium and spermatogenesis in the privileged survival of intratesticular grafts. J Urol 1985; 134: 782 786 - PubMed
-
- Cameron DF, Whittington K, Schultz RE, Selawry HP. Successful islet/abdominal testis transplantation does not require Leydig cells. Transplantation 1990; 50: 649 653 - PubMed
-
- Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant 1993; 2: 123 129 - PubMed
-
- Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes 1997; 46: 317 322 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

