CYLD regulates keratinocyte differentiation and skin cancer progression in humans
- PMID: 21900959
- PMCID: PMC3186900
- DOI: 10.1038/cddis.2011.82
CYLD regulates keratinocyte differentiation and skin cancer progression in humans
Abstract
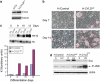
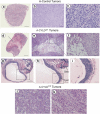
CYLD is a gene mutated in familial cylindromatosis and related diseases, leading to the development of skin appendages tumors. Although the deubiquitinase CYLD is a skin tumor suppressor, its role in skin physiology is unknown. Using skin organotypic cultures as experimental model to mimic human skin, we have found that CYLD acts as a regulator of epidermal differentiation in humans through the JNK signaling pathway. We have determined the requirement of CYLD for the maintenance of epidermal polarity, keratinocyte differentiation and apoptosis. We show that CYLD overexpression increases keratinocyte differentiation while CYLD loss of function impairs epidermal differentiation. In addition, we describe the important role of CYLD in the control of human non-melanoma skin cancer progression. Our results show the reversion of the malignancy of human squamous cell carcinomas that express increased levels of CYLD, while its functional inhibition enhances the aggressiveness of these tumors which progress toward spindle cell carcinomas. We have found that the mechanisms through which CYLD regulates skin cancer progression include the control of tumor differentiation, angiogenesis and cell survival. These findings of the role of CYLD in human skin cancer prognosis make our results relevant from a therapeutic point of view, and open new avenues for exploring novel cancer therapies.
Figures





References
-
- Gazel A, Banno T, Walsh R, Blumenberg M. Inhibition of JNK promotes differentiation of epidermal keratinocytes. J Biol Chem. 2006;281:20530–20541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

