Imaging gene delivery in a mouse model of congenital neuronal ceroid lipofuscinosis
- PMID: 21900963
- PMCID: PMC3235265
- DOI: 10.1038/gt.2011.118
Imaging gene delivery in a mouse model of congenital neuronal ceroid lipofuscinosis
Erratum in
- Gene Ther. 2012 Nov;19(11):1121
Abstract
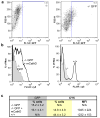
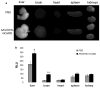
Adeno-associated virus (AAV)-mediated gene replacement for lysosomal disorders have been spurred by the ability of some serotypes to efficiently transduce neurons in the brain and by the ability of lysosomal enzymes to cross-correct among cells. Here, we explored enzyme replacement therapy in a knock-out mouse model of congenital neuronal ceroid lipofuscinosis (NCL), the most severe of the NCLs in humans. The missing protease in this disorder, cathepsin D (CathD) has high levels in the central nervous system. This enzyme has the potential advantage for assessing experimental therapy in that it can be imaged using a near-infrared fluorescence (NIRF) probe activated by CathD. Injections of an AAV2/rh8 vector-encoding mouse CathD (mCathD) into both cerebral ventricles and peritoneum of newborn knock-out mice resulted in a significant increase in lifespan. Successful delivery of active CathD by the AAV2/rh8-mCathD vector was verified by NIRF imaging of mouse embryonic fibroblasts from knock-out mice in culture, as well as by ex vivo NIRF imaging of the brain and liver after gene transfer. These studies support the potential effectiveness and imaging evaluation of enzyme replacement therapy to the brain and other organs in CathD null mice via AAV-mediated gene delivery in neonatal animals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Enzyme replacement therapy with recombinant pro-CTSD (cathepsin D) corrects defective proteolysis and autophagy in neuronal ceroid lipofuscinosis.Autophagy. 2020 May;16(5):811-825. doi: 10.1080/15548627.2019.1637200. Epub 2019 Jul 16. Autophagy. 2020. PMID: 31282275 Free PMC article.
-
Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis.Ann Neurol. 2012 Jun;71(6):797-804. doi: 10.1002/ana.23545. Epub 2012 Feb 24. Ann Neurol. 2012. PMID: 22368049 Free PMC article.
-
Partial correction of the CNS lysosomal storage defect in a mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal CNS administration of an adeno-associated virus serotype rh.10 vector expressing the human CLN3 gene.Hum Gene Ther. 2014 Mar;25(3):223-39. doi: 10.1089/hum.2012.253. Epub 2014 Mar 4. Hum Gene Ther. 2014. PMID: 24372003 Free PMC article.
-
Neuronal Ceroid Lipofuscinosis-Concepts, Classification, and Avenues for Therapy.CNS Neurosci Ther. 2025 Feb;31(2):e70261. doi: 10.1111/cns.70261. CNS Neurosci Ther. 2025. PMID: 39925015 Free PMC article. Review.
-
CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis).Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:682-8. Pediatr Endocrinol Rev. 2016. PMID: 27491216 Review.
Cited by
-
Advances in the Treatment of Neuronal Ceroid Lipofuscinosis.Expert Opin Orphan Drugs. 2019;7(11):473-500. doi: 10.1080/21678707.2019.1684258. Epub 2019 Nov 27. Expert Opin Orphan Drugs. 2019. PMID: 33365208 Free PMC article.
-
Sustained normalization of neurological disease after intracranial gene therapy in a feline model.Sci Transl Med. 2014 Apr 9;6(231):231ra48. doi: 10.1126/scitranslmed.3007733. Sci Transl Med. 2014. PMID: 24718858 Free PMC article.
-
Targeting lysosomes in human disease: from basic research to clinical applications.Signal Transduct Target Ther. 2021 Nov 8;6(1):379. doi: 10.1038/s41392-021-00778-y. Signal Transduct Target Ther. 2021. PMID: 34744168 Free PMC article. Review.
-
Neuronal Ceroid Lipofuscinosis: Potential for Targeted Therapy.Drugs. 2021 Jan;81(1):101-123. doi: 10.1007/s40265-020-01440-7. Drugs. 2021. PMID: 33242182 Review.
-
From Molecular Therapies to Lysosomal Transplantation and Targeted Drug Strategies: Present Applications, Limitations, and Future Prospects of Lysosomal Medications.Biomolecules. 2025 Feb 24;15(3):327. doi: 10.3390/biom15030327. Biomolecules. 2025. PMID: 40149863 Free PMC article. Review.
References
-
- Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther. 2006;6:227–241. - PubMed
-
- Vellodi A. Lysosomal storage disorders. Br J Haematol. 2005;128:413–431. - PubMed
-
- Fernandes Filho JA, Shapiro BE. Tay-Sachs disease. Arch Neurol. 2004;61:1466–1468. - PubMed
-
- Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–126. - PubMed
-
- Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–1445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

