Trypanothione reductase: a viable chemotherapeutic target for antitrypanosomal and antileishmanial drug design
- PMID: 21901070
- PMCID: PMC3155241
Trypanothione reductase: a viable chemotherapeutic target for antitrypanosomal and antileishmanial drug design
Abstract
Trypanosomiasis and leishmaniasis are two debilitating disease groups caused by parasites of Trypanosoma and Leishmania spp. and affecting millions of people worldwide. A brief outline of the potential targets for rational drug design against these diseases are presented, with an emphasis placed on the enzyme trypanothione reductase. Trypanothione reductase was identified as unique to parasites and proposed to be an effective target against trypanosomiasis and leishmaniasis. The biochemical basis of selecting this enzyme as a target, with reference to the simile and contrast to human analogous enzyme glutathione reductase, and the structural aspects of its active site are presented. The process of designing selective inhibitors for the enzyme trypanothione reductase has been discussed. An overview of the different chemical classes of inhibitors of trypanothione reductase with their inhibitory activities against the parasites and their prospects as future chemotherapeutic agents are briefly revealed.
Keywords: Chagas disease; Trypanothione; glutathione; rational drug design; sleeping sickness.
Figures







Similar articles
-
Mini review on tricyclic compounds as an inhibitor of trypanothione reductase.J Pharm Bioallied Sci. 2014 Oct;6(4):222-8. doi: 10.4103/0975-7406.142943. J Pharm Bioallied Sci. 2014. PMID: 25400403 Free PMC article. Review.
-
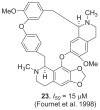
Phenothiazine inhibitors of trypanothione reductase as potential antitrypanosomal and antileishmanial drugs.J Med Chem. 1998 Jan 15;41(2):148-56. doi: 10.1021/jm960814j. J Med Chem. 1998. PMID: 9457238
-
Trypanothione as a target in the design of antitrypanosomal and antileishmanial agents.Curr Pharm Des. 2001 Aug;7(12):1117-41. doi: 10.2174/1381612013397564. Curr Pharm Des. 2001. PMID: 11472257 Review.
-
Peptoid inhibition of trypanothione reductase as a potential antitrypanosomal and antileishmanial drug lead.Amino Acids. 2002 Jun;22(4):297-308. doi: 10.1007/s007260200016. Amino Acids. 2002. PMID: 12107758
-
The parasite-specific trypanothione metabolism of trypanosoma and leishmania.Biol Chem. 2003 Apr;384(4):539-49. doi: 10.1515/BC.2003.062. Biol Chem. 2003. PMID: 12751784 Review.
Cited by
-
Chemotherapy of chronic indeterminate Chagas disease: a novel approach to treatment.Parasitol Res. 2008 Aug;103(3):663-9. doi: 10.1007/s00436-008-1029-x. Epub 2008 May 31. Parasitol Res. 2008. PMID: 18512075
-
Quinolizidine-Derived Lucanthone and Amitriptyline Analogues Endowed with Potent Antileishmanial Activity.Pharmaceuticals (Basel). 2020 Oct 25;13(11):339. doi: 10.3390/ph13110339. Pharmaceuticals (Basel). 2020. PMID: 33113777 Free PMC article.
-
In Vivo anti-trypanosomal activity of dichloromethane and methanol crude leaf extracts of Dovyalis abyssinica (Salicaceae) against Trypanosoma congolense.BMC Complement Altern Med. 2015 Aug 14;15:278. doi: 10.1186/s12906-015-0809-y. BMC Complement Altern Med. 2015. PMID: 26271481 Free PMC article.
-
Future Prospects in the Treatment of Parasitic Diseases: 2-Amino-1,3,4-Thiadiazoles in Leishmaniasis.Molecules. 2019 Apr 19;24(8):1557. doi: 10.3390/molecules24081557. Molecules. 2019. PMID: 31010226 Free PMC article. Review.
-
Repositioned Drugs for Chagas Disease Unveiled via Structure-Based Drug Repositioning.Int J Mol Sci. 2020 Nov 20;21(22):8809. doi: 10.3390/ijms21228809. Int J Mol Sci. 2020. PMID: 33233837 Free PMC article.
References
-
- Austin SE, Khan MOF, Douglas KT. Rational drug design using trypanothione reductase as a target for antitrypanosomal and anti-leishmanial drug leads. Drug Des Discov. 1999;16:5–23. - PubMed
-
- Bailey S, Smith K, Fairlamb AH, et al. Substrate interactions between trypanothione reductase and N1-glutathionylspermidine disulphide at 0.28-nm resolution. Eur. J. Biochem./FEBS. 1993;213:67–75. - PubMed
-
- Baillet S, Buisine E, Horvath D, et al. 2-Amino diphenylsulfides as inhibitors of trypanothione reductase: modification of the side chain. Bioorg Med Chem. 1996;4:891–9. - PubMed
-
- Bi X, Lopez C, Bacchi CJ, et al. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg Med Chem Lett. 2006;16:3229–32. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

